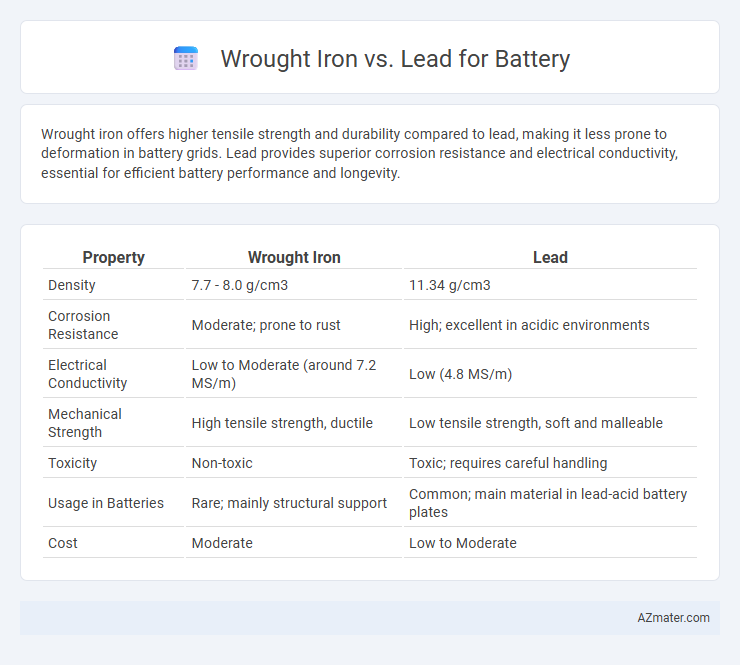
Wrought iron offers higher tensile strength and durability compared to lead, making it less prone to deformation in battery grids. Lead provides superior corrosion resistance and electrical conductivity, essential for efficient battery performance and longevity.
Table of Comparison
| Property | Wrought Iron | Lead |
|---|---|---|
| Density | 7.7 - 8.0 g/cm3 | 11.34 g/cm3 |
| Corrosion Resistance | Moderate; prone to rust | High; excellent in acidic environments |
| Electrical Conductivity | Low to Moderate (around 7.2 MS/m) | Low (4.8 MS/m) |
| Mechanical Strength | High tensile strength, ductile | Low tensile strength, soft and malleable |
| Toxicity | Non-toxic | Toxic; requires careful handling |
| Usage in Batteries | Rare; mainly structural support | Common; main material in lead-acid battery plates |
| Cost | Moderate | Low to Moderate |
Introduction to Battery Materials
Wrought iron and lead serve distinct roles in battery technology due to their differing electrochemical properties. Lead is predominantly used in lead-acid batteries as the primary material for electrodes, offering excellent conductivity and corrosion resistance essential for efficient charge storage and discharge cycles. Wrought iron, while historically significant in various industrial applications, lacks the electrochemical stability required for modern battery electrodes and is therefore rarely utilized in battery material composition.
Overview of Wrought Iron in Batteries
Wrought iron is known for its excellent mechanical strength and corrosion resistance, making it a durable alternative for battery components, especially in lead-acid batteries. Its granular structure provides enhanced conductivity and structural integrity, which helps improve battery lifespan and performance. Compared to lead, wrought iron offers a more environmentally friendly option due to its recyclability and lower toxicity.
The Role of Lead in Battery Technology
Lead plays a critical role in battery technology, particularly in lead-acid batteries, where it functions as the primary material for both the anode and cathode due to its excellent electrochemical properties. Its high density and corrosion resistance enable efficient energy storage and long cycle life, making it ideal for automotive and backup power applications. Wrought iron lacks the electrochemical stability and capacity of lead, rendering it unsuitable for modern battery technology.
Material Properties: Wrought Iron vs Lead
Wrought iron offers high tensile strength and excellent corrosion resistance, making it durable under mechanical stress, while lead is dense and highly malleable, providing superior electrochemical properties essential for efficient battery performance. Lead's low melting point and excellent conductivity enhance charge retention and cycling stability in batteries, whereas wrought iron's superior structural integrity supports robust battery casing and framework. Material choice impacts battery longevity and efficiency, with lead favored for internal electrode components and wrought iron for supportive, corrosion-resistant structural parts.
Electrochemical Performance Comparison
Wrought iron exhibits higher electrical conductivity and improved corrosion resistance in battery applications compared to lead, enhancing overall electrochemical efficiency. Lead's higher density and slower reaction kinetics result in increased internal resistance and reduced charge-discharge rates in lead-acid batteries. Electrochemical tests demonstrate wrought iron's superior cycle stability and faster electron transfer, making it a promising alternative for sustainable energy storage solutions.
Durability and Lifespan Analysis
Wrought iron exhibits high tensile strength and corrosion resistance, contributing to a longer lifespan in battery applications compared to lead, which is prone to sulfation and grid corrosion over time. Lead's softness enables easy manufacturing but reduces durability under cyclic stress and heat exposure, often resulting in shorter battery service life. Material choice significantly impacts battery longevity, with wrought iron-based components offering enhanced durability and sustained performance in demanding conditions.
Environmental Impact and Sustainability
Wrought iron, known for its durability and recyclability, has a lower environmental impact compared to lead, which poses significant ecological risks due to its toxicity and potential for soil and water contamination from battery disposal. Lead batteries require careful handling and recycling processes to mitigate hazards, while wrought iron components contribute less to environmental pollution throughout their lifecycle. Sustainability efforts favor materials like wrought iron that support circular economy principles, reducing dependency on hazardous substances and promoting safer battery technologies.
Cost Efficiency of Wrought Iron vs Lead
Wrought iron offers a cost-efficient alternative to lead in battery applications due to its lower raw material expenses and increased durability, which reduces replacement frequency and maintenance costs. Lead batteries typically involve higher toxicity-related handling and recycling costs, impacting overall lifecycle expenses compared to wrought iron options. The superior corrosion resistance and structural strength of wrought iron contribute to long-term savings, making it economically advantageous in industrial battery manufacturing.
Safety Considerations in Battery Applications
Wrought iron offers superior mechanical strength and corrosion resistance compared to lead, reducing the risk of structural failure in battery casings under high-stress conditions. Lead, while highly conductive and dense, poses significant safety concerns due to its toxicity and potential for environmental contamination in case of leakage. Proper handling and disposal protocols are critical when using lead in batteries to mitigate health hazards and ensure regulatory compliance.
Future Prospects and Technological Innovations
Wrought iron, traditionally valued for its durability, is increasingly being replaced by advanced lead alloys in battery manufacturing due to lead's superior electrochemical properties and recyclability. Emerging technologies such as lead-carbon hybrid electrodes enhance battery lifespan and efficiency, positioning lead-based batteries as practical solutions for future energy storage demands. Continued innovation in additive manufacturing and nano-engineering promises to optimize lead battery performance, while the environmental challenges associated with wrought iron make lead a more sustainable choice for next-generation batteries.
Infographic: Wrought iron vs Lead for Battery
 azmater.com
azmater.com