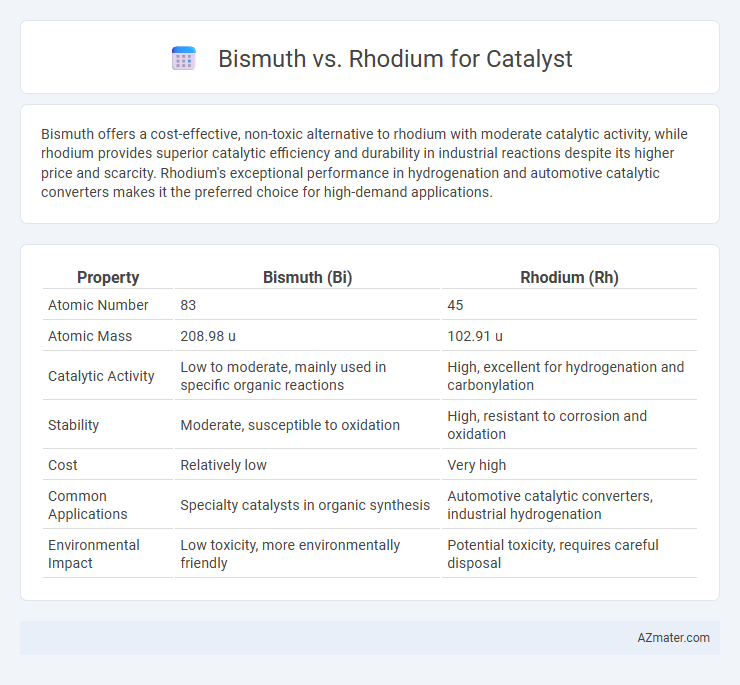
Bismuth offers a cost-effective, non-toxic alternative to rhodium with moderate catalytic activity, while rhodium provides superior catalytic efficiency and durability in industrial reactions despite its higher price and scarcity. Rhodium's exceptional performance in hydrogenation and automotive catalytic converters makes it the preferred choice for high-demand applications.
Table of Comparison
| Property | Bismuth (Bi) | Rhodium (Rh) |
|---|---|---|
| Atomic Number | 83 | 45 |
| Atomic Mass | 208.98 u | 102.91 u |
| Catalytic Activity | Low to moderate, mainly used in specific organic reactions | High, excellent for hydrogenation and carbonylation |
| Stability | Moderate, susceptible to oxidation | High, resistant to corrosion and oxidation |
| Cost | Relatively low | Very high |
| Common Applications | Specialty catalysts in organic synthesis | Automotive catalytic converters, industrial hydrogenation |
| Environmental Impact | Low toxicity, more environmentally friendly | Potential toxicity, requires careful disposal |
Introduction to Bismuth and Rhodium Catalysts
Bismuth catalysts offer a non-toxic, cost-effective alternative with unique Lewis acid properties, facilitating selective organic transformations such as oxidation and coupling reactions. Rhodium catalysts are renowned for their exceptional activity and selectivity in hydrogenation, hydroformylation, and carbon-carbon bond formation, driven by their versatile coordination chemistry and strong metal-ligand interactions. Both metals exhibit distinct catalytic behaviors influenced by their electronic structures, making them critical choices in green chemistry and industrial synthesis.
Chemical Properties Relevant to Catalysis
Bismuth exhibits low toxicity and high resistance to oxidation, making it useful in selective catalysis, particularly for environmental applications. Rhodium, characterized by its exceptional catalytic activity and stability, excels in hydrogenation and oxidation reactions due to its ability to activate molecular hydrogen and oxygen. The distinct electronic structures of bismuth and rhodium influence their catalytic efficiency, with rhodium providing superior turnover frequencies in industrial catalytic converters.
Mechanisms of Catalytic Action: Bismuth vs Rhodium
Bismuth catalysts typically operate via Lewis acid mechanisms, facilitating electrophilic activation of substrates through the empty 6p orbitals, which enhances selectivity in organic transformations such as oxidation and hydroamination. Rhodium catalysts proceed through oxidative addition and reductive elimination steps, leveraging variable oxidation states (Rh(I)/Rh(III)) to enable efficient C-H activation and hydrogenation reactions. The differing electronic structures of bismuth and rhodium dictate their unique catalytic pathways, with bismuth favoring soft Lewis acid interactions and rhodium enabling redox cycling for complex bond activation.
Efficiency and Selectivity in Catalytic Reactions
Bismuth and rhodium differ significantly in catalytic efficiency and selectivity, with rhodium exhibiting superior activity and high selectivity in hydrogenation and carbon-carbon coupling reactions due to its unique electronic structure. Bismuth, while less efficient in catalytic turnover, offers distinct selectivity advantages in oxidation reactions and environmentally benign processes, often enhancing catalyst stability and reducing toxicity. Rhodium's high cost and scarcity limit its large-scale applications, whereas bismuth's abundance presents a cost-effective alternative for specific catalytic systems emphasizing sustainability and selectivity.
Environmental Impact and Toxicity
Bismuth serves as an environmentally friendly catalyst due to its low toxicity and high biodegradability, making it safer for use in green chemistry and pharmaceutical applications. Rhodium, while highly efficient as a catalyst in automotive and industrial processes, poses significant environmental risks because of its scarcity and potential toxicity, necessitating careful handling and disposal. The choice between bismuth and rhodium catalysts hinges on balancing catalytic performance with environmental sustainability and human health safety.
Cost and Availability of Bismuth and Rhodium
Bismuth is significantly more abundant and cost-effective compared to rhodium, making it a preferred choice for large-scale catalytic applications where budget constraints exist. Rhodium, a rare and precious metal, commands a high price due to its limited availability and extensive demand in automotive catalytic converters. The disparity in cost and availability influences industrial decisions, with bismuth offering a sustainable alternative and rhodium reserved for applications requiring exceptional catalytic performance despite its expense.
Industrial Applications and Case Studies
Bismuth and rhodium exhibit distinct catalytic properties critical for industrial applications, with rhodium favored in high-performance catalytic converters due to its superior efficiency in reducing nitrogen oxides and its resilience under harsh conditions. Bismuth catalysts demonstrate enhanced selectivity in organic synthesis and environmental remediation, offering a cost-effective, non-toxic alternative in chemical manufacturing and wastewater treatment processes. Case studies reveal rhodium's dominance in automotive emission control technologies, while bismuth's role expands in green chemistry initiatives, emphasizing sustainable industrial practices.
Stability and Reusability of Catalysts
Bismuth catalysts exhibit moderate stability under mild reaction conditions but tend to degrade or leach in highly acidic or oxidative environments, limiting their long-term reusability. Rhodium catalysts demonstrate superior thermal and chemical stability due to their robust electronic structure, maintaining catalytic activity over multiple cycles without significant loss of performance. The high durability and resistance to deactivation make rhodium the preferred choice for industrial applications requiring continuous catalyst recycling.
Recent Advances in Catalyst Research
Recent advances in catalyst research highlight Bismuth's growing prominence due to its low toxicity and cost-effectiveness compared to Rhodium, traditionally favored for high-performance catalytic processes. Bismuth-based catalysts have shown superior selectivity and stability in oxidation and carbon-carbon coupling reactions, driven by novel nanostructured designs and doping techniques. Meanwhile, Rhodium continues to lead in hydrogenation and automotive catalytic converters, valued for its unmatched activity and resistance to sintering under harsh reaction conditions.
Future Prospects and Emerging Trends
Bismuth-based catalysts demonstrate promising future prospects due to their low toxicity, abundance, and unique electronic properties that enable efficient selective oxidation and reduction reactions, increasingly valued in sustainable chemistry. Rhodium catalysts maintain critical importance in automotive catalytic converters and hydrogenation processes, with emerging trends focusing on reducing Rhodium loading through alloying and nanostructuring to enhance activity and cost-efficiency. Advances in catalyst design harness bimetallic systems combining Bismuth and Rhodium for synergistic effects, improving catalytic performance and durability in environmental and industrial applications.
Infographic: Bismuth vs Rhodium for Catalyst
 azmater.com
azmater.com