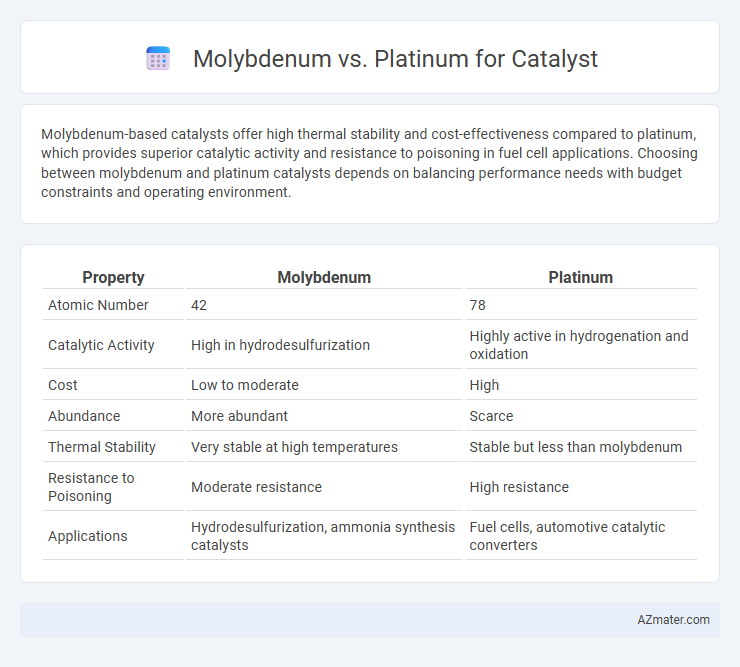
Molybdenum-based catalysts offer high thermal stability and cost-effectiveness compared to platinum, which provides superior catalytic activity and resistance to poisoning in fuel cell applications. Choosing between molybdenum and platinum catalysts depends on balancing performance needs with budget constraints and operating environment.
Table of Comparison
| Property | Molybdenum | Platinum |
|---|---|---|
| Atomic Number | 42 | 78 |
| Catalytic Activity | High in hydrodesulfurization | Highly active in hydrogenation and oxidation |
| Cost | Low to moderate | High |
| Abundance | More abundant | Scarce |
| Thermal Stability | Very stable at high temperatures | Stable but less than molybdenum |
| Resistance to Poisoning | Moderate resistance | High resistance |
| Applications | Hydrodesulfurization, ammonia synthesis catalysts | Fuel cells, automotive catalytic converters |
Introduction to Molybdenum and Platinum Catalysts
Molybdenum catalysts exhibit excellent thermal stability and resistance to sulfur poisoning, making them ideal for hydrodesulfurization and selective hydrogenation processes in the petrochemical industry. Platinum catalysts are highly valued for their superior catalytic activity and selectivity in applications such as automotive catalytic converters and fuel cells due to their ability to efficiently facilitate oxidation and reduction reactions. Both metals offer unique electronic structures and surface properties that influence reaction pathways, with molybdenum often preferred for cost-effective heterogeneous catalysis and platinum favored for high-performance catalytic systems.
Chemical Properties Comparison
Molybdenum exhibits a high melting point of 2623degC and variable oxidation states ranging from -II to +VI, which facilitates diverse catalytic reactions, especially in hydrodesulfurization processes. Platinum, with a melting point of 1768degC, predominantly shows oxidation states of 0, +2, and +4, offering exceptional stability and activity in hydrogenation and oxidation catalysts. The differing electronic configurations and surface chemistries of molybdenum (4d^5 5s^1) and platinum (5d^9 6s^1) directly impact their adsorption energies and catalytic selectivity in industrial applications.
Catalytic Efficiency and Performance
Molybdenum catalysts exhibit exceptional catalytic efficiency, particularly in hydrodesulfurization processes due to their sulfur tolerance and cost-effectiveness compared to platinum. Platinum catalysts offer superior catalytic performance in oxidation and hydrogenation reactions, benefiting from high activity and selectivity but at a significantly higher cost. The choice between molybdenum and platinum depends on the specific reaction environment, where molybdenum excels in harsh, sulfur-containing conditions and platinum in precision catalytic applications requiring rapid and selective transformations.
Cost and Availability Analysis
Molybdenum offers a significant cost advantage over platinum, as its lower market price and greater abundance reduce overall catalyst expenses. The widespread availability of molybdenum, primarily sourced from large mining operations, ensures a stable supply chain compared to platinum, which is rarer and heavily concentrated in fewer geographic regions. These factors make molybdenum a more economically viable choice for large-scale catalytic applications without compromising performance in certain reactions.
Environmental Impact and Sustainability
Molybdenum catalysts exhibit lower environmental impact compared to platinum due to their greater abundance and reduced mining emissions, supporting more sustainable industrial processes. Platinum mining and refining generate significant ecological disturbances and higher carbon footprints, challenging long-term sustainability goals. Molybdenum's recyclability and corrosion resistance further enhance catalyst lifecycle sustainability in various chemical applications.
Industrial Applications
Molybdenum exhibits superior performance in high-temperature industrial catalysis, particularly in hydrodesulfurization processes for refining petroleum, due to its excellent resistance to sulfur poisoning and cost-effectiveness. Platinum catalysts, widely used in automotive catalytic converters and fuel cells, demonstrate exceptional activity and selectivity for oxidation and hydrogenation reactions under milder conditions, though they are limited by higher costs and susceptibility to poisoning by contaminants like sulfur and carbon monoxide. Both metals play crucial roles in chemical manufacturing and pollution control, with molybdenum favored for bulk industrial applications and platinum preferred for high-value, precision catalytic processes.
Resistance to Deactivation and Poisoning
Molybdenum catalysts exhibit notable resistance to deactivation and poisoning, especially in sulfur-rich and high-temperature environments, due to their strong metal-support interactions and ability to form stable surface oxides. Platinum catalysts, while highly active, are more susceptible to poisoning by sulfur, carbon monoxide, and other impurities, leading to faster deactivation in harsh conditions. The durability of molybdenum makes it preferable for processes requiring long-term catalyst stability against fouling and sintering.
Longevity and Durability
Molybdenum catalysts exhibit exceptional longevity due to their high melting point (2623degC) and resistance to corrosion in harsh industrial environments, outperforming platinum in high-temperature applications. Platinum offers superior durability and catalytic efficiency at lower temperatures but tends to degrade faster under extreme conditions because of sintering and poisoning effects. The choice between molybdenum and platinum catalysts depends on operational longevity requirements and environmental stress factors, with molybdenum favored for sustained performance in aggressive chemical processes.
Recent Innovations and Research
Recent innovations in molybdenum catalysts highlight enhanced hydrogen evolution and nitrogen fixation efficiencies through nanostructured molybdenum sulfide and carbide composites. Platinum catalysts maintain dominance in fuel cell applications due to superior electron transfer rates, but research explores alloying with molybdenum to reduce platinum loading while preserving catalytic activity. Advanced studies on molybdenum-platinum bimetallic catalysts reveal synergistic effects that improve durability and selectivity in hydrogen fuel production and chemical synthesis.
Choosing the Right Catalyst: Key Considerations
Molybdenum offers excellent resistance to high temperatures and sulfur poisoning, making it suitable for hydrodesulfurization catalysts in refining processes. Platinum provides superior catalytic activity and selectivity in oxidation and hydrogenation reactions but is more expensive and less tolerant to certain poisons. Choosing the right catalyst depends on factors such as reaction conditions, cost constraints, and the desired balance between activity and durability.
Infographic: Molybdenum vs Platinum for Catalyst
 azmater.com
azmater.com