Aluminum offers a lightweight, corrosion-resistant alternative to lead in battery production, enhancing energy density and recyclability. Lead batteries provide higher conductivity and are cost-effective but suffer from heavier weight and environmental toxicity.
Table of Comparison
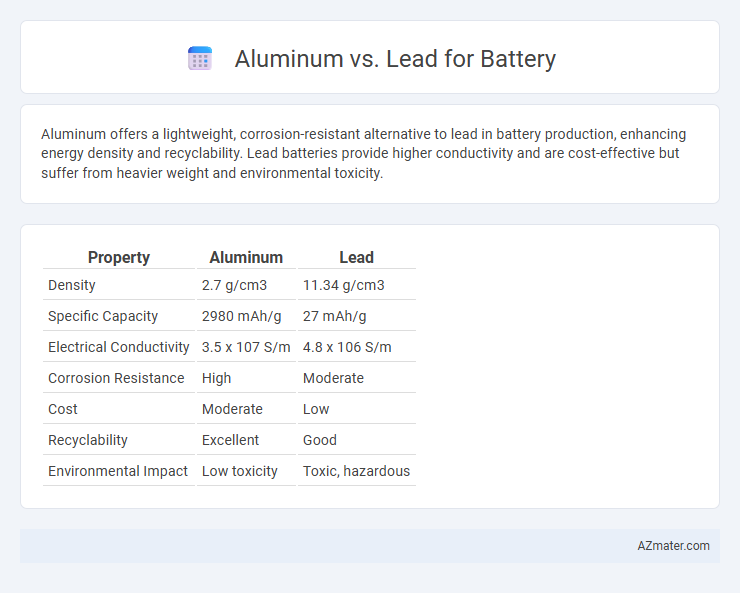
| Property | Aluminum | Lead |
|---|---|---|
| Density | 2.7 g/cm3 | 11.34 g/cm3 |
| Specific Capacity | 2980 mAh/g | 27 mAh/g |
| Electrical Conductivity | 3.5 x 107 S/m | 4.8 x 106 S/m |
| Corrosion Resistance | High | Moderate |
| Cost | Moderate | Low |
| Recyclability | Excellent | Good |
| Environmental Impact | Low toxicity | Toxic, hazardous |
Introduction to Battery Materials: Aluminum vs Lead
Aluminum and lead serve as key materials in battery technology, each offering distinct electrochemical properties that influence performance and application. Aluminum batteries provide benefits such as lightweight composition, higher energy density, and enhanced corrosion resistance compared to traditional lead-acid batteries. Lead remains widely used for its cost-effectiveness, established recycling infrastructure, and reliable conductivity, primarily in automotive and stationary power applications.
Historical Overview of Lead and Aluminum Batteries
Lead-acid batteries, invented in 1859 by Gaston Plante, dominated early energy storage due to their reliable deep-cycle capability and affordability, primarily used in automotive and industrial applications. Aluminum batteries emerged in the late 20th century as a promising alternative, exploiting aluminum's high theoretical capacity, abundance, and low cost for innovative energy storage solutions. Despite aluminum's advantages, lead-acid technology remains widespread due to its mature manufacturing infrastructure and recycling systems.
Chemical Properties and Electrochemical Performance
Aluminum offers a higher theoretical capacity (2980 mAh/g) compared to lead (75 mAh/g) due to its trivalent charge, enhancing energy density in battery applications. Its electrochemical potential (-1.66 V vs SHE) enables efficient electron transfer but poses challenges with passivation and corrosion, requiring specialized electrolytes. Lead's stable electrochemical behavior, lower specific capacity, and well-established Pb-PbO2 redox chemistry contribute to its widespread use in lead-acid batteries despite inferior energy density and higher weight.
Energy Density Comparison
Aluminum batteries typically offer higher volumetric energy density compared to lead-acid batteries, enabling more energy storage in a smaller footprint. Lead-acid batteries, however, have higher gravimetric energy density due to lead's substantial mass, but they are limited by lower overall energy efficiency and shorter cycle life. Advances in aluminum-ion technology show potential for surpassing lead-acid energy density metrics while improving charge rates and lifespan.
Environmental Impact and Sustainability
Aluminum batteries offer significant environmental advantages over lead batteries due to aluminum's high abundance and recyclability, minimizing resource depletion and landfill waste. Lead batteries pose greater ecological risks, including toxic lead contamination and hazardous waste challenges, which require stringent disposal regulations. Sustainable battery development increasingly favors aluminum technology to reduce environmental footprint while maintaining energy efficiency.
Cost Analysis: Manufacturing and Material Sourcing
Aluminum offers significant cost advantages over lead in battery manufacturing due to its abundance and lower raw material price per ton, which reduces material sourcing expenses by up to 35%. The manufacturing process for aluminum batteries benefits from easier recyclability and reduced handling hazards, leading to lower operational costs compared to lead-based batteries that require extensive safety measures and environmental compliance. Economies of scale in aluminum production further decrease total manufacturing costs, making aluminum-based batteries a more cost-effective solution for large-scale energy storage applications.
Battery Lifespan and Maintenance Requirements
Aluminum batteries typically offer a longer lifespan compared to lead-acid batteries due to their higher resistance to corrosion and reduced sulfation issues, leading to extended cycle life and more reliable performance. Lead batteries require regular maintenance such as checking electrolyte levels and cleaning terminals to prevent sulfation and corrosion, which can significantly impact battery longevity. Aluminum batteries are generally maintenance-free, making them a more convenient option with lower ownership costs over time.
Applications and Industry Adoption
Aluminum batteries are increasingly favored in electric vehicle and grid storage applications due to their lightweight nature and higher energy density compared to lead-based batteries. Lead-acid batteries dominate the automotive starter market and backup power systems because of their cost-effectiveness and mature recycling infrastructure. Industrial adoption trends reveal a shift toward aluminum batteries in renewable energy and portable electronics sectors driven by safety, environmental benefits, and longer lifecycle performance.
Safety Considerations and Hazards
Aluminum batteries offer enhanced safety due to their lower toxicity and reduced risk of heavy metal contamination compared to traditional lead-acid batteries. Lead batteries pose significant hazards including lead poisoning, environmental contamination, and potential acid spills that require careful handling and disposal protocols. Fire risk is generally lower in aluminum batteries, while lead batteries can produce explosive hydrogen gas during charging, necessitating proper ventilation and monitoring.
Future Trends and Innovations in Battery Technology
Aluminum batteries are gaining traction due to their higher abundance, lower cost, and superior recyclability compared to lead-acid batteries, driving future trends toward sustainable energy storage. Innovations in aluminum-ion technology focus on enhancing energy density and charging speed, promising longer lifecycle and faster recharge times suited for electric vehicles and grid storage. Meanwhile, research on lead-acid batteries centers on improving sulfuric acid gel electrolytes and expanding hybrid designs to boost performance and reduce environmental impact.
Infographic: Aluminum vs Lead for Battery
 azmater.com
azmater.com