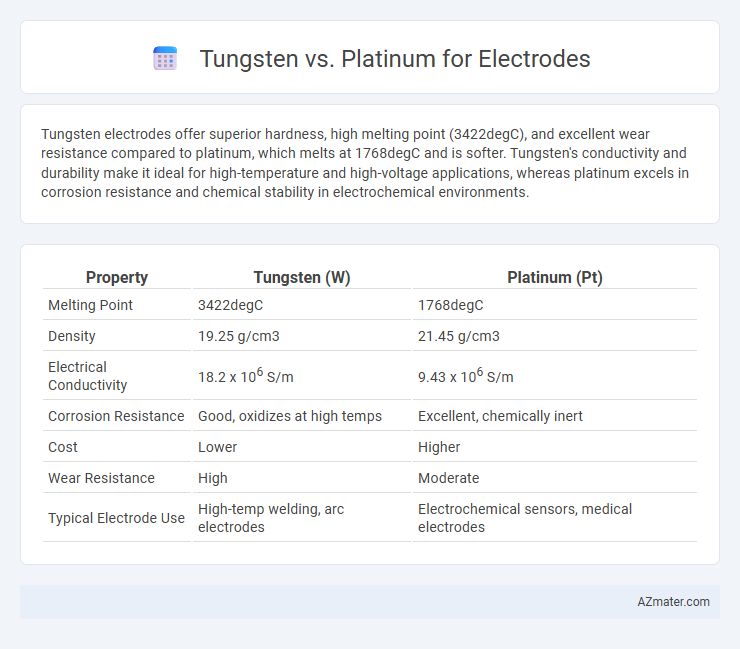
Tungsten electrodes offer superior hardness, high melting point (3422degC), and excellent wear resistance compared to platinum, which melts at 1768degC and is softer. Tungsten's conductivity and durability make it ideal for high-temperature and high-voltage applications, whereas platinum excels in corrosion resistance and chemical stability in electrochemical environments.
Table of Comparison
| Property | Tungsten (W) | Platinum (Pt) |
|---|---|---|
| Melting Point | 3422degC | 1768degC |
| Density | 19.25 g/cm3 | 21.45 g/cm3 |
| Electrical Conductivity | 18.2 x 106 S/m | 9.43 x 106 S/m |
| Corrosion Resistance | Good, oxidizes at high temps | Excellent, chemically inert |
| Cost | Lower | Higher |
| Wear Resistance | High | Moderate |
| Typical Electrode Use | High-temp welding, arc electrodes | Electrochemical sensors, medical electrodes |
Introduction to Electrode Materials
Tungsten and platinum are widely used electrode materials due to their distinct electrochemical properties and stability. Tungsten offers high melting point, excellent hardness, and good conductivity, making it suitable for high-temperature applications and durable electrode tips. Platinum provides superior corrosion resistance, excellent catalytic activity, and chemical inertness, which enhances electrode longevity and performance in sensitive electrochemical processes.
Overview of Tungsten and Platinum
Tungsten exhibits exceptional hardness, a high melting point of 3422degC, and excellent electrical conductivity, making it ideal for durable electrode applications in harsh environments. Platinum offers superior corrosion resistance, excellent conductivity, and stability under high temperatures up to 1768degC, which enhances performance in electrodes exposed to corrosive or oxidative conditions. Both metals provide unique advantages: tungsten is preferred for mechanical strength and thermal endurance, while platinum excels in chemical stability and longevity in electrochemical systems.
Electrical Conductivity Comparison
Tungsten exhibits electrical conductivity of approximately 18.2 x 10^6 S/m, whereas platinum's conductivity is around 9.43 x 10^6 S/m, making tungsten nearly twice as conductive as platinum. The higher conductivity of tungsten contributes to lower electrical resistance and improved efficiency in electrode applications. However, platinum offers superior chemical stability and oxidation resistance, factors which may influence electrode performance despite its lower conductivity.
Corrosion Resistance and Durability
Tungsten electrodes offer superior corrosion resistance due to their high melting point and chemical stability, making them ideal for harsh environments. Platinum electrodes, while also resistant to corrosion, provide exceptional durability with excellent resistance to oxidative degradation and mechanical wear. Comparing both, tungsten excels in high-temperature applications, whereas platinum is preferred for longevity in electrochemical processes due to its unmatched inertness.
Cost Analysis: Tungsten vs Platinum
Tungsten electrodes cost significantly less than platinum, making them a more economical choice for applications requiring durability and high melting points. Despite platinum's superior corrosion resistance and conductivity, its high price limits its use to specialized, high-performance electrodes where cost is less critical. Tungsten provides a cost-effective balance of strength and longevity in general electrode manufacturing without compromising essential performance.
Thermal Stability and Operating Temperatures
Tungsten electrodes exhibit superior thermal stability with a melting point of 3422degC, making them ideal for high-temperature applications such as arc welding and high-power electrical contacts. Platinum electrodes, with a melting point of 1768degC, offer excellent corrosion resistance but have lower thermal stability compared to tungsten, limiting their use in extreme temperature environments. Tungsten's ability to maintain structural integrity at operating temperatures above 3000degC ensures enhanced durability and performance under thermal stress.
Electrochemical Performance
Tungsten electrodes exhibit excellent electrochemical stability with high resistance to corrosion and oxidation, making them ideal for prolonged use in harsh electrolyte environments. Platinum electrodes provide superior catalytic activity and exceptional conductivity, enhancing electron transfer rates and reducing overpotential in electrochemical reactions. While tungsten offers durability and cost-effectiveness, platinum's enhanced electrochemical performance makes it preferred for applications requiring high sensitivity and efficiency.
Application Areas and Industry Use
Tungsten electrodes are widely used in welding, electronics, and aerospace industries due to their exceptional hardness, high melting point, and excellent electrical conductivity. Platinum electrodes find extensive application in chemical processing, medical devices, and fuel cells because of their superior corrosion resistance, stability in harsh environments, and biocompatibility. Both metals serve critical roles but are selected based on the specific operational environment, with tungsten preferred for high-temperature and mechanical durability applications and platinum favored in chemically aggressive or biomedical contexts.
Maintenance and Longevity
Tungsten electrodes offer exceptional durability and require minimal maintenance due to their high melting point and resistance to erosion, making them ideal for long-term use in welding applications. Platinum electrodes, while corrosion-resistant and stable at high temperatures, generally exhibit shorter lifespan and need more frequent inspection and upkeep to maintain performance. Choosing tungsten over platinum often results in lower maintenance costs and enhanced electrode longevity in industrial settings.
Environmental and Safety Considerations
Tungsten electrodes exhibit high melting points and superior durability, reducing the frequency of replacements and minimizing metal waste, which supports environmental sustainability. Platinum electrodes, while more chemically inert and corrosion-resistant, pose higher environmental costs due to platinum mining and refinement processes with significant ecological impacts. Safety considerations favor tungsten as it emits fewer hazardous particles during use, whereas platinum electrodes require careful handling to prevent costly damage and environmental contamination.
Infographic: Tungsten vs Platinum for Electrode
 azmater.com
azmater.com