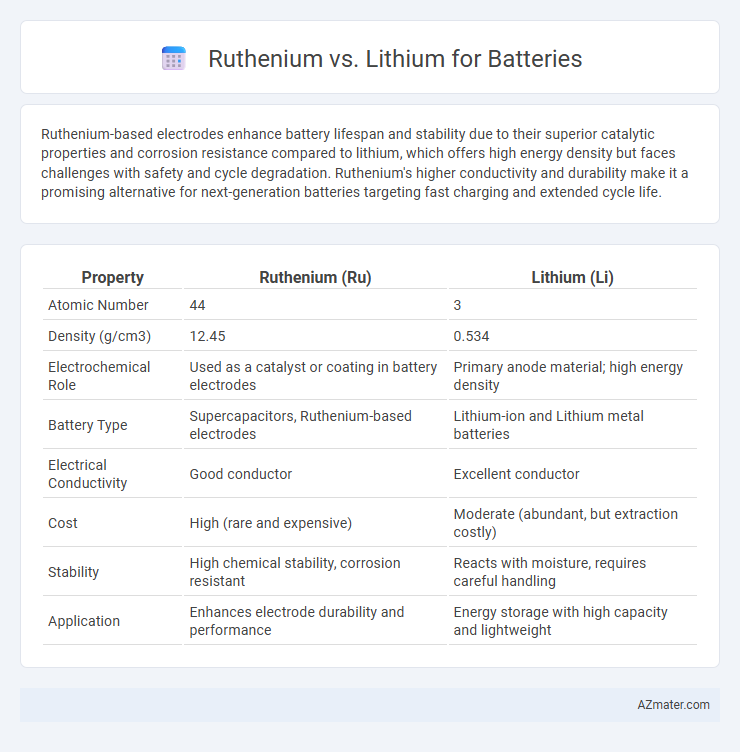
Ruthenium-based electrodes enhance battery lifespan and stability due to their superior catalytic properties and corrosion resistance compared to lithium, which offers high energy density but faces challenges with safety and cycle degradation. Ruthenium's higher conductivity and durability make it a promising alternative for next-generation batteries targeting fast charging and extended cycle life.
Table of Comparison
| Property | Ruthenium (Ru) | Lithium (Li) |
|---|---|---|
| Atomic Number | 44 | 3 |
| Density (g/cm3) | 12.45 | 0.534 |
| Electrochemical Role | Used as a catalyst or coating in battery electrodes | Primary anode material; high energy density |
| Battery Type | Supercapacitors, Ruthenium-based electrodes | Lithium-ion and Lithium metal batteries |
| Electrical Conductivity | Good conductor | Excellent conductor |
| Cost | High (rare and expensive) | Moderate (abundant, but extraction costly) |
| Stability | High chemical stability, corrosion resistant | Reacts with moisture, requires careful handling |
| Application | Enhances electrode durability and performance | Energy storage with high capacity and lightweight |
Introduction to Ruthenium and Lithium in Batteries
Ruthenium and lithium serve distinct roles in battery technology, with lithium being a widely used metal in rechargeable lithium-ion batteries due to its high energy density and light weight. Ruthenium, a rare transition metal, is often employed as a catalyst or electrode material in advanced battery systems, enhancing charge efficiency and cycle life. While lithium primarily drives energy storage capacity, ruthenium contributes to performance optimization and longevity in next-generation battery applications.
Chemical Properties and Electrochemical Behavior
Ruthenium exhibits high electrical conductivity and excellent catalytic properties, making it valuable in enhancing battery electrode performance through improved charge transfer and stability. Lithium, characterized by its low atomic weight and high electrochemical potential, serves as a primary ion carrier in rechargeable batteries, enabling high energy density and rapid charge-discharge cycles. The contrasting chemical reactivity and electrode potentials of ruthenium and lithium influence battery efficiency, with ruthenium often used as an additive to improve electrode durability and lithium as the essential active element in lithium-ion battery systems.
Energy Density Comparison
Ruthenium-based batteries offer significantly higher energy density compared to lithium-ion batteries, reaching up to 400 Wh/kg versus lithium's typical 150-250 Wh/kg, making them ideal for applications requiring extended power storage. The higher energy density of ruthenium enables longer battery life and reduced weight in electric vehicles and portable electronics. However, cost and material availability remain challenges for large-scale adoption compared to lithium's well-established supply chain.
Cost and Material Abundance
Ruthenium, a rare and expensive platinum-group metal, significantly increases battery costs due to its limited global availability and complex extraction processes. Lithium, abundant in resources such as spodumene and brine deposits, remains the more cost-effective choice for battery manufacturing despite recent price fluctuations caused by high demand. The scarcity of ruthenium leads to supply constraints, making lithium the preferred material in large-scale energy storage applications where cost and material abundance are critical factors.
Battery Performance and Efficiency
Ruthenium-based electrodes in batteries demonstrate superior catalytic activity and stability, enabling higher charge-discharge efficiency compared to lithium-only counterparts. Lithium batteries excel in energy density but face limitations in cycle life and thermal stability, where ruthenium-enhanced systems offer improved conductivity and faster reaction kinetics. Integrating ruthenium into lithium battery designs enhances overall battery performance by reducing internal resistance and increasing capacity retention over extended cycles.
Safety and Stability Considerations
Ruthenium-based cathodes in batteries offer enhanced thermal stability and reduced risk of thermal runaway compared to lithium metal anodes, which are prone to dendrite formation and short-circuiting. Ruthenium's high melting point and robust chemical inertness contribute to longer cycle life and safer operation under high current densities. In contrast, lithium's reactivity with electrolytes necessitates advanced protective measures like solid-state electrolytes or coatings to ensure safety and maintain stability during charge-discharge cycles.
Environmental Impact and Sustainability
Ruthenium, a rare and expensive transition metal, offers high energy density and long cycle life in battery applications but faces challenges due to its limited availability and extensive mining environmental impacts, including habitat disruption and toxic waste. Lithium, more abundant and widely used in rechargeable batteries, also presents environmental concerns such as water-intensive extraction, soil degradation, and pollution from battery disposal and recycling processes. Sustainable battery development increasingly prioritizes reducing reliance on rare metals like ruthenium while improving lithium extraction methods and enhancing recycling technologies to minimize ecological footprint.
Current Applications and Research Developments
Ruthenium is primarily used as a catalyst in lithium-ion battery electrodes, enhancing charge capacity and cycle stability, while lithium serves as the fundamental ion transporting charge contributing to high energy density. Recent research focuses on ruthenium-based compounds like ruthenium oxides to improve battery performance in supercapacitors and hybrid applications, leveraging their excellent electrical conductivity and electrochemical stability. In contrast, lithium continues to dominate battery commercialization with ongoing advancements targeting lithium-metal and solid-state lithium batteries to increase safety and energy efficiency.
Market Trends and Future Prospects
Ruthenium, with its high electrochemical stability and conductivity, is gaining attention for enhancing lithium-ion battery cathodes, potentially improving energy density and cycle life. Lithium remains the dominant ion for rechargeable batteries, driven by widespread demand in electric vehicles and consumer electronics, fueling ongoing market growth forecasted to reach over $100 billion by 2030. Emerging research on ruthenium-based electrode materials suggests promising future prospects for hybrid battery technologies that combine ruthenium's performance benefits with lithium's market dominance.
Conclusion: Choosing Between Ruthenium and Lithium
Ruthenium offers exceptional catalytic properties and enhanced battery lifespan, making it ideal for high-performance and specialized energy storage applications. Lithium remains the dominant choice due to its abundance, cost-effectiveness, and proven efficiency in large-scale commercial batteries. Selecting between ruthenium and lithium depends on balancing budget constraints with performance requirements, as ruthenium excels in niche markets while lithium suits widespread, cost-sensitive energy solutions.
Infographic: Ruthenium vs Lithium for Battery
 azmater.com
azmater.com