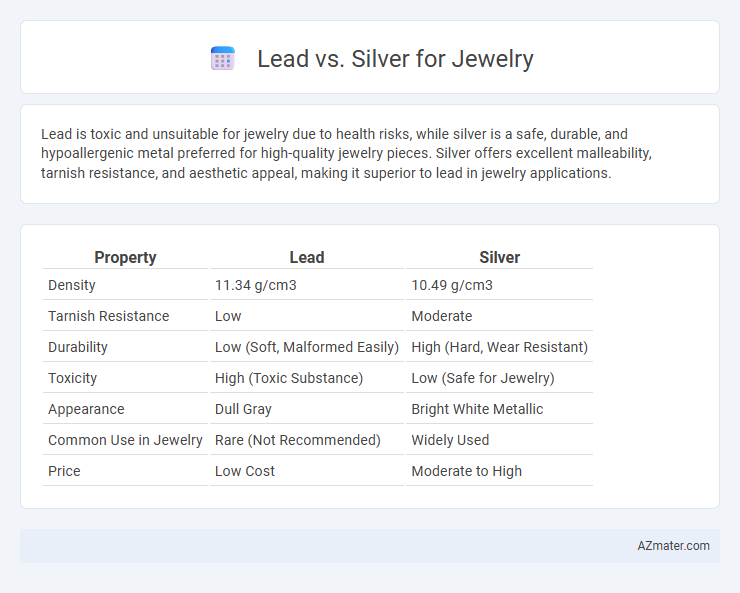
Lead is toxic and unsuitable for jewelry due to health risks, while silver is a safe, durable, and hypoallergenic metal preferred for high-quality jewelry pieces. Silver offers excellent malleability, tarnish resistance, and aesthetic appeal, making it superior to lead in jewelry applications.
Table of Comparison
| Property | Lead | Silver |
|---|---|---|
| Density | 11.34 g/cm3 | 10.49 g/cm3 |
| Tarnish Resistance | Low | Moderate |
| Durability | Low (Soft, Malformed Easily) | High (Hard, Wear Resistant) |
| Toxicity | High (Toxic Substance) | Low (Safe for Jewelry) |
| Appearance | Dull Gray | Bright White Metallic |
| Common Use in Jewelry | Rare (Not Recommended) | Widely Used |
| Price | Low Cost | Moderate to High |
Introduction: Lead vs Silver in Jewelry
Silver is a highly favored metal in jewelry due to its durability, lustrous appearance, and hypoallergenic properties, making it suitable for everyday wear. Lead, by contrast, is rarely used in fine jewelry because of its toxicity, heavy weight, and susceptibility to tarnishing, posing health risks with prolonged skin contact. Jewelry makers prioritize silver for its safe composition and elegant aesthetic, ensuring both beauty and wearer safety.
Historical Use of Lead and Silver in Jewelry
Lead has been used historically in jewelry due to its malleability and low melting point, allowing intricate designs and affordable production, especially in ancient civilizations such as the Romans and Egyptians. Silver, prized for its durability, luster, and resistance to corrosion, has long been favored for fine jewelry and ceremonial pieces throughout history, with extensive use in Greek, Roman, and medieval European artifacts. Archaeological findings reveal lead-based alloys often served as inexpensive substitutes or decorative components, while silver's enduring value and symbolic significance ensured its continued prominence in jewelry craftsmanship.
Chemical Properties of Lead and Silver
Lead exhibits a dense atomic structure with a high atomic number of 82 and is chemically characterized by its softness, low melting point (327.5degC), and tendency to oxidize forming a stable oxide layer. Silver, with an atomic number of 47, is a highly conductive metal prized for its resistance to corrosion and oxidation, maintaining its lustrous appearance due to its strong electron affinity and low reactivity with atmospheric gases. The chemical inertness of silver contrasts with lead's reactivity, making silver more suitable for durable jewelry that retains shine without tarnishing.
Health Risks: Lead vs Silver
Lead in jewelry poses significant health risks due to its toxic properties, potentially causing lead poisoning, neurological damage, and developmental issues upon prolonged skin contact or accidental ingestion. Silver, especially sterling silver, is generally safe and hypoallergenic, minimizing adverse reactions and long-term health hazards. Jewelry containing lead is often banned or strictly regulated to prevent heavy metal exposure, while silver remains a preferred choice for safe, durable adornments.
Durability and Longevity Compared
Silver offers superior durability and longevity compared to lead, which is a soft, malleable metal prone to bending and damage over time. Sterling silver, an alloy containing 92.5% silver and 7.5% copper, enhances strength and resistance to wear, making it ideal for everyday jewelry use. Unlike lead, which can tarnish quickly and deteriorate, silver maintains its shine longer and withstands daily wear better, ensuring lasting beauty and structural integrity.
Allergic Reactions and Skin Sensitivity
Lead in jewelry often causes allergic reactions such as contact dermatitis and skin irritation due to its toxic properties, making it unsuitable for sensitive skin. Silver, especially sterling silver, is hypoallergenic and widely preferred for people with skin sensitivities because it rarely triggers allergic responses. Choosing silver jewelry reduces the risk of skin allergies and provides a safer option for regular wear.
Aesthetic Differences: Appearance and Shine
Lead jewelry exhibits a heavier, more muted luster with a soft, grayish tone that absorbs light differently, resulting in a subtler shine compared to silver. Silver jewelry offers a bright, reflective gleam with a cool white hue that enhances intricate designs through its high polish and mirror-like finish. The distinct appearance of silver jewelry makes it ideal for pieces requiring a vibrant, eye-catching shine, while lead's aesthetic suits designs emphasizing weight and a vintage or industrial look.
Cost Comparison: Affordability of Lead vs Silver
Lead is significantly more affordable than silver due to its abundant availability and lower extraction costs. Silver's market price is much higher, driven by its rarity and demand in fine jewelry, making it a premium choice. For budget-conscious consumers, lead offers a cost-effective alternative, though it lacks the durability and aesthetic appeal of silver.
Environmental Impact of Lead and Silver Mining
Lead mining releases toxic heavy metals and sulfur dioxide, causing soil and water contamination that severely harms local ecosystems and human health. Silver mining involves extensive use of cyanide and mercury, which can lead to long-lasting water pollution and habitat destruction if not properly managed. Both metals pose significant environmental risks, but lead's toxicity and persistence in the environment make its impact particularly hazardous for surrounding communities.
Choosing the Right Metal for Your Jewelry
Choosing the right metal for your jewelry involves weighing factors like durability, appearance, and safety. Silver offers a bright, lustrous finish and is hypoallergenic, making it ideal for everyday wear, while lead, due to its toxicity and heaviness, is unsuitable for jewelry. Opting for silver ensures a balance of elegance, longevity, and health-conscious wearability.
Infographic: Lead vs Silver for Jewelry
 azmater.com
azmater.com