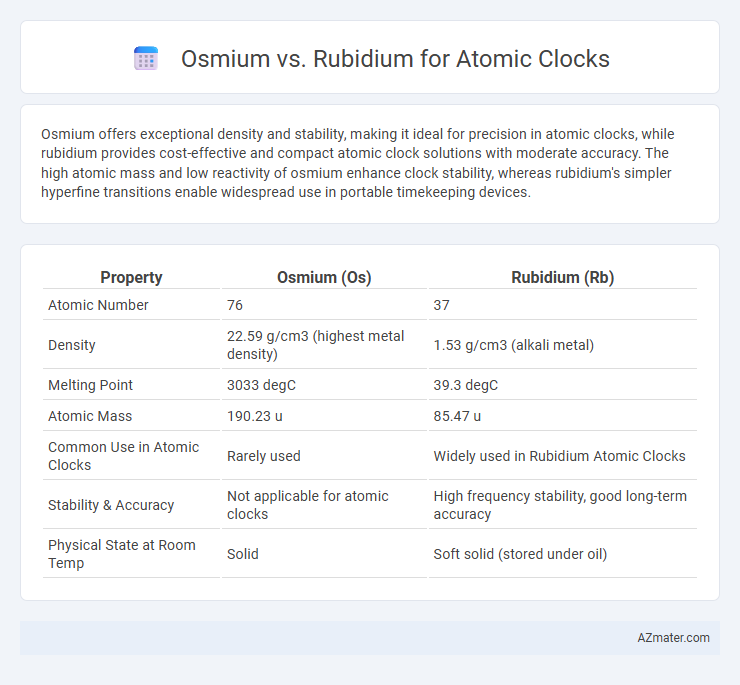
Osmium offers exceptional density and stability, making it ideal for precision in atomic clocks, while rubidium provides cost-effective and compact atomic clock solutions with moderate accuracy. The high atomic mass and low reactivity of osmium enhance clock stability, whereas rubidium's simpler hyperfine transitions enable widespread use in portable timekeeping devices.
Table of Comparison
| Property | Osmium (Os) | Rubidium (Rb) |
|---|---|---|
| Atomic Number | 76 | 37 |
| Density | 22.59 g/cm3 (highest metal density) | 1.53 g/cm3 (alkali metal) |
| Melting Point | 3033 degC | 39.3 degC |
| Atomic Mass | 190.23 u | 85.47 u |
| Common Use in Atomic Clocks | Rarely used | Widely used in Rubidium Atomic Clocks |
| Stability & Accuracy | Not applicable for atomic clocks | High frequency stability, good long-term accuracy |
| Physical State at Room Temp | Solid | Soft solid (stored under oil) |
Introduction to Atomic Clocks
Atomic clocks rely on the precise frequency of electron transitions within atoms to measure time with extraordinary accuracy. Osmium, with its complex electron structure, offers highly stable atomic transitions but is less commonly used due to technical challenges in laser cooling and state detection. Rubidium, on the other hand, is widely employed in atomic clocks because of its simpler electronic configuration, ease of manipulation using optical methods, and reliable hyperfine transition frequency that enables compact and cost-effective timekeeping devices.
The Role of Elements in Atomic Clock Precision
Osmium's dense electron cloud and high atomic number contribute to extremely stable energy levels, enhancing atomic clock precision through reduced quantum noise. Rubidium, widely used in atomic clocks, offers well-characterized hyperfine transitions with accessible microwave frequencies, balancing accuracy and practical implementation. The choice between osmium and rubidium impacts the clock's stability, frequency accuracy, and susceptibility to environmental perturbations, making elemental properties critical to timekeeping performance.
Osmium: Properties Relevant to Atomic Timekeeping
Osmium's exceptional density and stable isotopic composition make it a promising candidate for atomic timekeeping, as its atomic transitions exhibit minimal variation under environmental changes. The element's high atomic number (76) and low vapor pressure contribute to precise frequency standards essential for atomic clocks. These properties enable enhanced accuracy and stability compared to lighter alkali metals like Rubidium, commonly used in current atomic clock technology.
Rubidium: Characteristics and Applications in Clocks
Rubidium atomic clocks exploit the hyperfine transition frequency of rubidium-87 atoms, offering high stability and accuracy at a relatively low cost, making them ideal for telecommunications and GPS systems. Rubidium clocks operate at microwave frequencies around 6.834 GHz, providing precise timekeeping without the complexity and expense of cesium or hydrogen maser standards. Their compact size, low power consumption, and robustness enable widespread use in network synchronization, satellite navigation, and scientific research where reliable timing is essential.
Frequency Standards: Osmium vs Rubidium
Osmium offers superior frequency stability and precision due to its highly stable nuclear magnetic resonance properties, making it a promising candidate for advanced atomic clocks. Rubidium, widely used in commercial atomic clocks, provides reliable frequency standards with moderate accuracy and long-term stability through its hyperfine transitions in the rubidium-87 isotope. The comparison highlights osmium's potential for next-generation ultra-precise frequency standards, while rubidium remains a cost-effective and practical solution for many timing applications.
Stability and Accuracy: A Comparative Analysis
Osmium and rubidium serve distinct roles in atomic clock technology, with rubidium clocks recognized for their high stability and accuracy in timekeeping due to the precise microwave transitions of rubidium-87 atoms. Osmium, while less common in atomic clock applications, possesses exceptional density and electron configuration properties that could theoretically enhance frequency stability but lacks extensive practical implementation data. Rubidium atomic clocks exhibit fractional frequency stability on the order of 10^-12, making them reliable for navigation and telecommunications, whereas osmium-based systems remain experimental with potential future improvements in long-term stability and accuracy.
Practical Considerations: Availability and Cost
Rubidium atomic clocks are widely used due to their relatively low cost and high availability, making them practical for commercial and scientific applications. Osmium, despite its high density and unique atomic properties, is rare and expensive, limiting its feasibility for widespread atomic clock use. The abundant supply and affordable price of rubidium enable scalable production and maintenance of precise timekeeping devices.
Applications of Osmium-Based Atomic Clocks
Osmium-based atomic clocks offer exceptional precision in timekeeping, making them ideal for advanced scientific research, deep space navigation, and synchronization of global positioning systems (GPS). The high stability and minimal frequency drift of osmium isotopes enhance measurements in fundamental physics experiments and improve the accuracy of telecommunications networks. Unlike rubidium clocks, osmium clocks provide superior performance in environments requiring ultra-precise timing over extended periods.
Rubidium Clocks in Modern Technology
Rubidium atomic clocks are widely used in modern technology due to their compact size, reliability, and cost-effectiveness compared to osmium-based alternatives. These clocks leverage the hyperfine transition of rubidium-87 atoms to achieve precise timekeeping essential for GPS navigation, telecommunications, and network synchronization. Osmium, while having unique atomic properties, is less practical for atomic clocks because of its complexity and limited application in commercial systems.
Future Prospects: Which Element Leads Atomic Timekeeping?
Osmium's exceptional density and stable isotopes offer promising avenues for next-generation atomic clocks with ultra-high precision, while rubidium remains a widely used element due to its established technology and affordable optical transitions. Future atomic timekeeping could see osmium outperform rubidium by leveraging its superior atomic stability and reduced environmental susceptibility, potentially resulting in clocks with unprecedented accuracy and longevity. Research and development into quantum states of osmium may lead to breakthroughs that redefine precision standards and enhance global time synchronization networks.
Infographic: Osmium vs Rubidium for Atomic Clock
 azmater.com
azmater.com