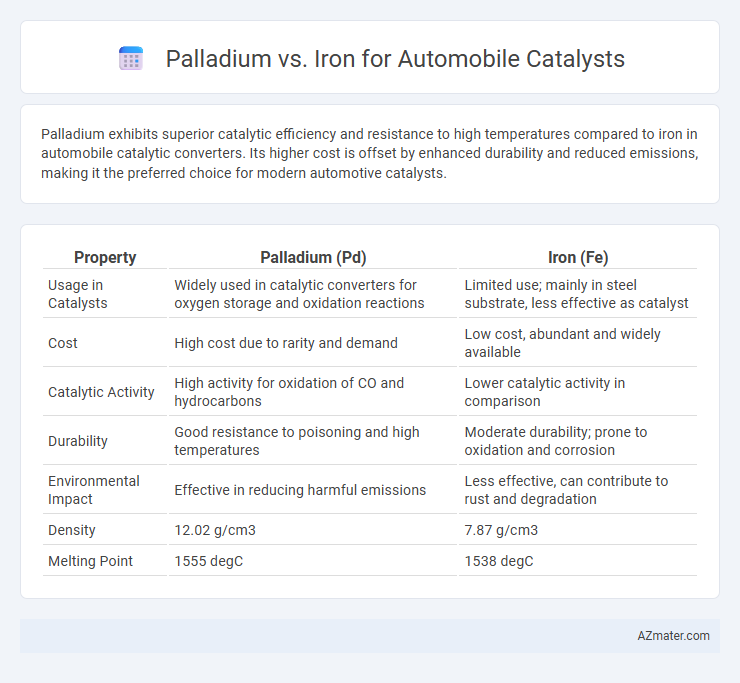
Palladium exhibits superior catalytic efficiency and resistance to high temperatures compared to iron in automobile catalytic converters. Its higher cost is offset by enhanced durability and reduced emissions, making it the preferred choice for modern automotive catalysts.
Table of Comparison
| Property | Palladium (Pd) | Iron (Fe) |
|---|---|---|
| Usage in Catalysts | Widely used in catalytic converters for oxygen storage and oxidation reactions | Limited use; mainly in steel substrate, less effective as catalyst |
| Cost | High cost due to rarity and demand | Low cost, abundant and widely available |
| Catalytic Activity | High activity for oxidation of CO and hydrocarbons | Lower catalytic activity in comparison |
| Durability | Good resistance to poisoning and high temperatures | Moderate durability; prone to oxidation and corrosion |
| Environmental Impact | Effective in reducing harmful emissions | Less effective, can contribute to rust and degradation |
| Density | 12.02 g/cm3 | 7.87 g/cm3 |
| Melting Point | 1555 degC | 1538 degC |
Introduction to Automobile Catalysts
Automobile catalysts primarily use palladium and iron as active metals to reduce harmful emissions from exhaust gases. Palladium offers superior catalytic efficiency in converting carbon monoxide, hydrocarbons, and nitrogen oxides into less toxic substances, making it a preferred choice in gasoline engines. Iron, often employed in diesel oxidation catalysts and selective catalytic reduction systems, serves as a cost-effective alternative with robust performance in reducing nitrogen oxides under specific conditions.
Importance of Catalysts in Emission Control
Palladium outperforms iron in automobile catalysts due to its superior ability to facilitate oxidation and reduction reactions critical for reducing harmful emissions. Catalysts like palladium enable the conversion of carbon monoxide, hydrocarbons, and nitrogen oxides into less toxic gases, significantly decreasing environmental pollution from vehicle exhaust. Efficient emission control with palladium-based catalysts is essential for meeting stringent regulatory standards and improving air quality.
Overview of Palladium and Iron as Catalyst Materials
Palladium and iron serve distinct roles in automobile catalysts, with palladium excelling in oxidation reactions due to its high catalytic activity and resistance to sulfur poisoning. Iron, although less active, is valued for its abundance and cost-effectiveness, often utilized in three-way catalysts for nitrogen oxide reduction through its redox capabilities. The choice between palladium and iron hinges on balancing performance demands with economic and environmental considerations in emissions control technologies.
Chemical Properties of Palladium vs Iron
Palladium exhibits superior catalytic activity due to its exceptional ability to adsorb and activate oxygen and hydrocarbons at lower temperatures, facilitating efficient oxidation and reduction reactions in automobile catalysts. Iron, while abundant and cost-effective, has lower catalytic efficiency and is more prone to oxidation under operational conditions, limiting its effectiveness in emission control. The unique electronic structure and resistance to sulfur poisoning make palladium highly preferred in three-way catalysts for converting harmful gases like CO, NOx, and hydrocarbons into less toxic substances.
Catalytic Efficiency in Automotive Applications
Palladium exhibits superior catalytic efficiency compared to iron in automotive catalytic converters due to its exceptional ability to facilitate oxidation and reduction reactions at lower temperatures, leading to more effective exhaust gas treatment. Palladium catalysts demonstrate higher durability and better resistance to sulfur poisoning, which ensures sustained performance in reducing harmful emissions such as CO, NOx, and hydrocarbons. Iron-based catalysts, while cost-effective, generally require higher temperatures to reach optimal activity and offer lower overall conversion rates, making palladium the preferred choice for modern automotive emissions control.
Cost Comparison: Palladium Versus Iron
Palladium catalysts in automobiles are significantly more expensive than iron-based alternatives due to the metal's rarity and high market demand. Iron, being abundant and cost-effective, offers a cheaper solution but may require larger quantities or advanced formulations to achieve similar catalytic performance. The cost disparity influences manufacturer choices, with palladium-driven catalysts dominating despite higher expenses because of superior efficiency and regulatory compliance benefits.
Environmental Impact and Sustainability
Palladium-based catalysts in automobiles offer higher efficiency in converting harmful gases like CO, NOx, and hydrocarbons into less toxic emissions compared to iron-based catalysts, making them more effective for stringent environmental regulations. Despite being more expensive and scarcer, palladium's superior catalytic performance contributes to lower overall emissions and improved fuel efficiency, supporting sustainability goals. Iron catalysts, while abundant and cost-effective, often exhibit lower durability and efficiency, leading to higher emissions and less sustainable long-term use in automotive applications.
Durability and Longevity in Real-World Conditions
Palladium-based catalysts exhibit superior durability and longevity in automobile catalytic converters under real-world conditions due to their higher resistance to thermal degradation and poisoning by sulfur and other contaminants. Iron-based catalysts, while cost-effective, tend to degrade faster and lose efficiency more quickly when exposed to high temperatures and exhaust pollutants. The enhanced stability of palladium ensures prolonged catalytic performance, contributing to lower emissions and reduced maintenance over the vehicle's lifespan.
Industrial Trends and Availability
Palladium remains the preferred metal for automobile catalysts due to its superior catalytic efficiency in reducing harmful emissions, supported by increasing demand in the automotive industry. Iron, while abundant and cost-effective, faces challenges in matching palladium's performance, leading to ongoing research in iron-based catalysts to provide sustainable alternatives. Industrial trends highlight a shift towards optimizing palladium use amidst supply constraints and rising prices, while iron catalysts are emerging as viable options for future eco-friendly vehicle technologies.
Future Perspectives: Innovations in Catalyst Technology
Palladium-based catalysts are experiencing increased demand due to their superior performance in reducing nitrogen oxides (NOx) and carbon monoxide (CO) emissions, driving innovations in more efficient and durable formulations. Iron catalysts, notably in non-noble metal catalysts (NNMCs), are gaining attention for cost-effective, sustainable alternatives in automotive exhaust treatment through advances in nanostructuring and active site optimization. Future perspectives highlight hybrid catalyst systems combining palladium's high activity with iron's abundance, aiming to achieve enhanced catalytic efficiency and reduced reliance on scarce precious metals in next-generation automobile emission controls.
Infographic: Palladium vs Iron for Automobile Catalyst
 azmater.com
azmater.com