Osmium offers exceptional density and durability, making it a promising yet costly material for high-performance battery electrodes. Cadmium, while cheaper and traditionally used in nickel-cadmium batteries, poses significant toxicity risks and lower energy density compared to osmium-based alternatives.
Table of Comparison
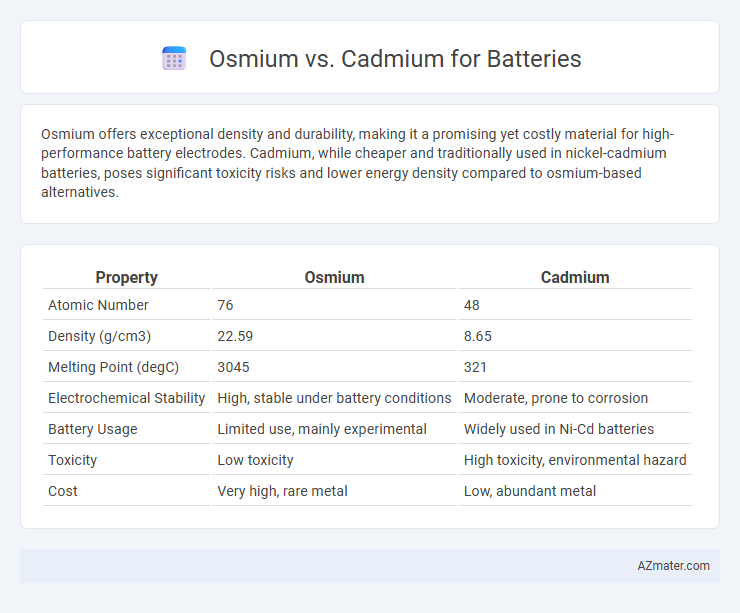
| Property | Osmium | Cadmium |
|---|---|---|
| Atomic Number | 76 | 48 |
| Density (g/cm3) | 22.59 | 8.65 |
| Melting Point (degC) | 3045 | 321 |
| Electrochemical Stability | High, stable under battery conditions | Moderate, prone to corrosion |
| Battery Usage | Limited use, mainly experimental | Widely used in Ni-Cd batteries |
| Toxicity | Low toxicity | High toxicity, environmental hazard |
| Cost | Very high, rare metal | Low, abundant metal |
Introduction to Osmium and Cadmium in Battery Technology
Osmium and cadmium are metals explored for battery technology due to their unique electrochemical properties, with cadmium widely used in nickel-cadmium (NiCd) rechargeable batteries known for high cycle stability and energy density. Osmium, a rare and dense platinum-group metal, offers promising catalytic efficiency and conductivity but is less common due to cost and rarity. Advances in battery research focus on optimizing cadmium's toxicity challenges and exploring osmium's potential for next-generation energy storage applications.
Chemical Properties: Osmium vs Cadmium
Osmium exhibits exceptional chemical stability and high density, making it resistant to corrosion and ideal for long-lasting battery electrodes, while cadmium is more reactive with moderate toxicity, commonly used in nickel-cadmium rechargeable batteries due to its ability to undergo stable redox reactions. Osmium's higher melting point (3045 degC) compared to cadmium's lower melting point (321 degC) influences thermal stability and battery lifespan under extreme conditions. The distinct oxidation states of osmium (+8 to 0) enable diverse electrochemical applications, whereas cadmium primarily operates in the +2 oxidation state, affecting charge capacity and cycle efficiency in battery cells.
Energy Density Comparison
Osmium offers a significantly higher energy density compared to cadmium, primarily due to its exceptional atomic weight and stability, making it a promising material for advanced battery applications. Cadmium-based batteries, such as nickel-cadmium (NiCd), have lower energy densities and suffer from memory effect issues, limiting their efficiency and cycle life. The enhanced energy density of osmium enables longer battery runtimes and improved charge retention, positioning it as a superior choice for high-performance energy storage solutions.
Recharge Cycles and Battery Lifespan
Osmium-based batteries typically offer higher recharge cycle stability compared to cadmium batteries, providing up to 1,500 recharge cycles without significant capacity loss. Cadmium batteries, such as nickel-cadmium (NiCd), generally support around 500 to 1,000 recharge cycles before performance degradation occurs. The enhanced durability of osmium electrodes contributes to a longer battery lifespan, making osmium a more sustainable choice for applications requiring frequent recharging.
Safety and Environmental Concerns
Osmium is significantly less toxic than cadmium, making it a safer choice for battery applications with lower risks of environmental contamination. Cadmium, widely known for its high toxicity and potential to cause severe health issues, poses substantial environmental hazards due to its persistence and bioaccumulation in ecosystems. Batteries utilizing osmium benefit from improved safety profiles and reduced environmental impact compared to traditional cadmium-based batteries.
Cost and Availability of Osmium and Cadmium
Osmium is an extremely rare and costly metal, making it economically unfeasible for widespread battery production, while cadmium is more abundant and significantly cheaper, commonly used in nickel-cadmium (NiCd) batteries despite environmental concerns. The limited supply and high price of osmium restrict its practicality in commercial battery manufacturing compared to cadmium, which benefits from established extraction and recycling processes. Cost efficiency and material availability heavily favor cadmium over osmium for battery applications.
Performance in Different Battery Chemistries
Osmium exhibits exceptional electrochemical stability and higher catalytic activity in battery electrodes, enhancing performance in lithium-ion and solid-state batteries. Cadmium, while historically used in nickel-cadmium (NiCd) batteries, offers moderate energy density but suffers from toxicity and lower cycle life compared to osmium-based materials. Osmium's superior conductivity and corrosion resistance make it a promising candidate for next-generation battery chemistries requiring high capacity and longevity.
Real-World Applications and Use Cases
Osmium and cadmium are metals with distinct roles in battery technology, where cadmium is primarily used in nickel-cadmium (NiCd) rechargeable batteries, known for their durability in power tools and emergency lighting systems. Osmium, being rare and expensive, lacks widespread use in batteries but offers potential in advanced catalytic electrodes for fuel cells due to its high density and corrosion resistance. Real-world applications favor cadmium for cost-effective, reliable energy storage, while osmium remains experimental in niche electrochemical systems requiring high performance.
Future Prospects and Innovations
Osmium holds promise in battery technology due to its exceptional density and durability, potentially enabling higher energy storage and longer lifespan compared to traditional materials. Cadmium, while historically used in nickel-cadmium batteries, faces declining popularity because of toxicity concerns and environmental regulations. Innovations in osmium-based electrodes and nanostructured materials suggest a future shift towards safer, more efficient batteries with enhanced charge capacity and stability.
Conclusion: Which Metal Holds More Promise for Batteries?
Osmium offers exceptional density and stability but is hindered by scarcity and high cost, limiting its practical application in batteries. Cadmium, while toxic, has proven utility in rechargeable battery technologies like NiCd batteries, demonstrating better commercial viability. Considering material availability, performance, and environmental impact, cadmium currently holds more promise for battery use than osmium.
Infographic: Osmium vs Cadmium for Battery
 azmater.com
azmater.com