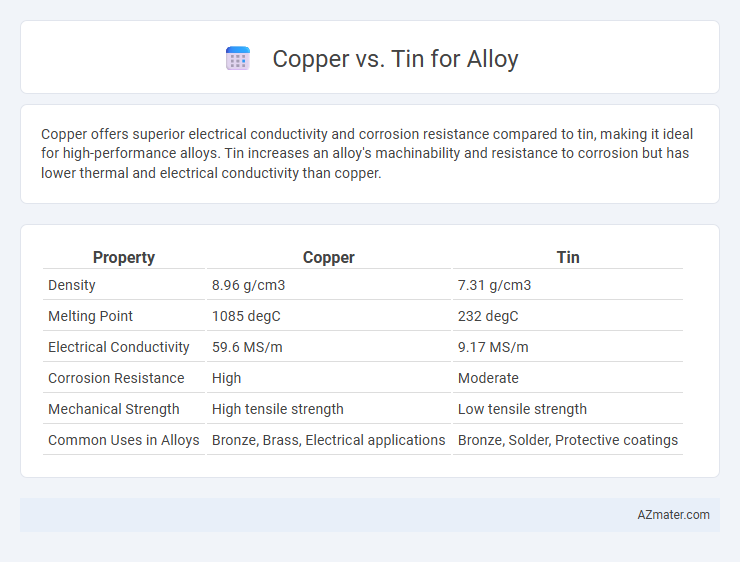
Copper offers superior electrical conductivity and corrosion resistance compared to tin, making it ideal for high-performance alloys. Tin increases an alloy's machinability and resistance to corrosion but has lower thermal and electrical conductivity than copper.
Table of Comparison
| Property | Copper | Tin |
|---|---|---|
| Density | 8.96 g/cm3 | 7.31 g/cm3 |
| Melting Point | 1085 degC | 232 degC |
| Electrical Conductivity | 59.6 MS/m | 9.17 MS/m |
| Corrosion Resistance | High | Moderate |
| Mechanical Strength | High tensile strength | Low tensile strength |
| Common Uses in Alloys | Bronze, Brass, Electrical applications | Bronze, Solder, Protective coatings |
Introduction to Copper and Tin in Alloy Production
Copper, known for its excellent electrical conductivity and corrosion resistance, serves as a primary metal in alloy production, especially in bronze and brass formulations where it enhances strength and durability. Tin, valued for its low melting point and resistance to oxidation, is commonly alloyed with copper to produce bronze, improving castability and wear resistance. The combination of copper and tin in alloys balances mechanical properties and corrosion resistance, making them essential in applications ranging from marine hardware to musical instruments.
Historical Significance of Copper and Tin Alloys
Copper and tin alloys, primarily bronze, played a pivotal role in advancing early human civilizations by enabling the creation of durable tools, weapons, and art. The discovery of bronze around 3300 BCE marked the beginning of the Bronze Age, leading to significant technological and cultural developments across Mesopotamia, Egypt, and the Indus Valley. The combined properties of copper and tin--copper's malleability and tin's hardness--resulted in an alloy that shaped historical progress in metallurgy and trade.
Chemical Properties: Copper vs Tin
Copper exhibits high electrical conductivity and corrosion resistance due to its atomic structure and stable oxidation states, primarily +1 and +2. Tin, known for its low toxicity and resistance to oxidation, predominantly displays +2 and +4 oxidation states, contributing to its use as a protective coating in alloys. The combination of copper's ductility and tin's brittleness results in bronze, an alloy with enhanced hardness and corrosion resistance, optimized for various industrial applications.
Mechanical Strength and Durability Comparison
Copper offers superior mechanical strength and enhanced durability compared to tin when used in alloys, contributing to improved resistance to wear and deformation under stress. Alloys with higher copper content, such as bronze, exhibit significant tensile strength and corrosion resistance, making them ideal for structural and industrial applications. Tin, while providing excellent corrosion resistance and malleability, lacks the robustness required for high-strength components, leading to a preference for copper-rich alloys in demanding mechanical environments.
Alloying Behavior and Compatibility
Copper exhibits excellent alloying behavior with tin, forming a range of bronze alloys characterized by superior hardness, corrosion resistance, and machinability. Tin acts as a solid solution strengthener in copper, improving wear resistance and reducing the metal's tendency to creep under stress. The compatibility of copper and tin at atomic levels enables the formation of homogeneous alloys with diverse applications in marine hardware, musical instruments, and coinage.
Corrosion Resistance in Alloys
Copper exhibits superior corrosion resistance in alloys compared to tin, especially in marine and industrial environments, due to its ability to form a protective oxide layer that prevents further oxidation. Tin enhances corrosion resistance primarily in tin-copper alloys like bronze, providing improved resistance against tarnishing and erosion in freshwater and mildly acidic conditions. Alloys with higher copper content typically demonstrate greater durability and longevity under corrosive stress than those with elevated tin proportions.
Industrial Applications of Copper and Tin Alloys
Copper-tin alloys, primarily bronze, exhibit excellent corrosion resistance, wear resistance, and strength, making them ideal for industrial applications such as marine hardware, bearings, and electrical connectors. Copper enhances thermal and electrical conductivity, while tin improves hardness and casts easily, providing a balanced alloy for machinery components and architectural fittings. Industries leverage these properties for durable, long-lasting products in automotive, aerospace, and construction sectors.
Cost and Availability Factors
Copper generally costs more than tin due to its higher demand in electrical applications and limited scarcity in certain regions. Tin is more abundant and easier to source, making it a cost-effective choice for alloy production. The price and availability of copper and tin directly influence the economic feasibility of their alloys, with copper alloys typically commanding a premium price in the market.
Environmental Impact and Sustainability
Copper and tin, commonly alloyed to form bronze, differ significantly in environmental impact and sustainability. Copper extraction causes habitat disruption and high energy consumption, but its recyclability reduces long-term environmental costs. Tin mining often leads to deforestation and water pollution, with limited recycling infrastructure posing sustainability challenges compared to copper.
Choosing the Right Metal for Your Alloy Needs
Copper offers excellent electrical conductivity and corrosion resistance, making it ideal for alloys requiring durability and high performance in electrical applications. Tin enhances alloy strength and corrosion resistance, especially in bronze alloys, making it suitable for decorative and marine uses. Selecting the right metal depends on the specific alloy properties desired, such as conductivity, strength, and environmental resistance.
Infographic: Copper vs Tin for Alloy
 azmater.com
azmater.com