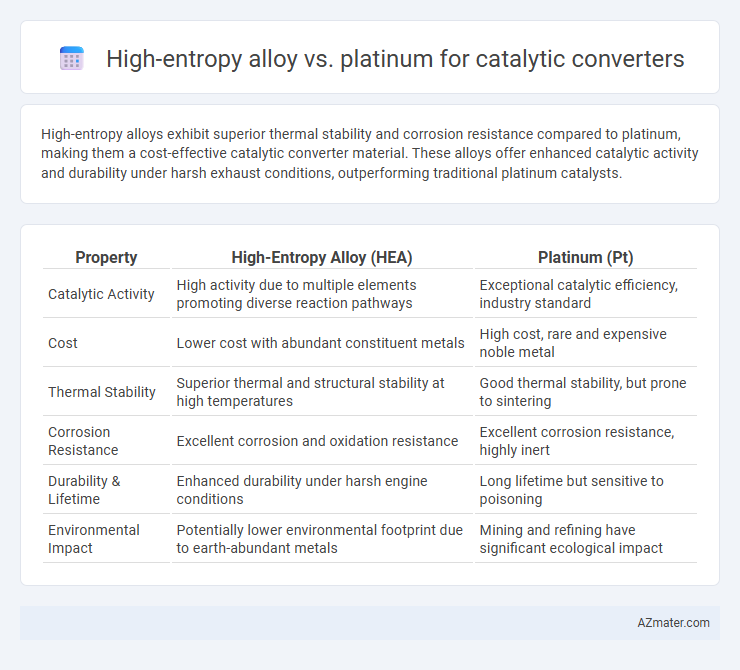
High-entropy alloys exhibit superior thermal stability and corrosion resistance compared to platinum, making them a cost-effective catalytic converter material. These alloys offer enhanced catalytic activity and durability under harsh exhaust conditions, outperforming traditional platinum catalysts.
Table of Comparison
| Property | High-Entropy Alloy (HEA) | Platinum (Pt) |
|---|---|---|
| Catalytic Activity | High activity due to multiple elements promoting diverse reaction pathways | Exceptional catalytic efficiency, industry standard |
| Cost | Lower cost with abundant constituent metals | High cost, rare and expensive noble metal |
| Thermal Stability | Superior thermal and structural stability at high temperatures | Good thermal stability, but prone to sintering |
| Corrosion Resistance | Excellent corrosion and oxidation resistance | Excellent corrosion resistance, highly inert |
| Durability & Lifetime | Enhanced durability under harsh engine conditions | Long lifetime but sensitive to poisoning |
| Environmental Impact | Potentially lower environmental footprint due to earth-abundant metals | Mining and refining have significant ecological impact |
Introduction: High-Entropy Alloys vs Platinum in Catalytic Converters
High-entropy alloys (HEAs) offer a promising alternative to traditional platinum-based catalysts in catalytic converters due to their unique multicomponent composition, which enhances catalytic activity and thermal stability. While platinum remains the benchmark for efficient exhaust gas conversion, HEAs demonstrate superior resistance to sintering and poisoning, potentially reducing material costs and improving long-term performance. Recent studies highlight that HEAs can achieve comparable or even enhanced catalytic efficiency in oxidation and reduction reactions crucial for emission control.
Material Composition and Structure
High-entropy alloys (HEAs) consist of multiple principal elements, typically five or more, combined in near-equiatomic ratios, resulting in a highly stable, single-phase solid solution with enhanced thermal stability and corrosion resistance compared to traditional platinum catalysts. Platinum, a noble metal with a face-centered cubic (FCC) structure, exhibits excellent catalytic activity but suffers from high cost and susceptibility to sintering under high temperatures, which reduces its long-term efficiency. The complex multi-element composition of HEAs offers tunable electronic structures and lattice distortions that improve catalytic performance and durability in catalytic converters beyond that of pure platinum.
Catalytic Efficiency and Reaction Mechanisms
High-entropy alloys (HEAs) exhibit enhanced catalytic efficiency in catalytic converters due to their unique multi-element composition, which promotes diverse active sites and synergistic effects that improve reaction pathways for pollutant oxidation and reduction. In comparison to platinum, HEAs demonstrate superior resistance to poisoning and thermal degradation, maintaining stable catalytic activity at higher temperatures. The complex surface structures of HEAs facilitate multiple reaction mechanisms simultaneously, leading to higher conversion rates of CO, NOx, and hydrocarbons compared to the relatively uniform catalytic action offered by platinum.
Cost Comparison and Economic Viability
High-entropy alloys (HEAs) offer a promising alternative to platinum in catalytic converters due to their significantly lower material costs and abundant elemental composition, reducing dependence on scarce precious metals. While platinum remains highly effective for catalytic efficiency, HEAs provide competitive catalytic activity with enhanced thermal stability and corrosion resistance, leading to longer service life and lower maintenance expenses. Economic viability improves as HEAs mitigate price volatility associated with platinum, enabling more sustainable and cost-effective large-scale deployment in automotive emissions control.
Thermal Stability and Durability
High-entropy alloys exhibit superior thermal stability compared to traditional platinum catalysts, maintaining structural integrity at elevated temperatures exceeding 1000degC due to their multi-element composition and lattice distortion effects. This enhanced thermal resilience translates into improved durability under harsh exhaust conditions, resisting sintering and phase degradation that commonly reduce platinum catalyst lifespan. Consequently, high-entropy alloys offer a promising alternative for catalytic converters by combining prolonged operational stability with robust resistance to thermal and chemical stresses.
Resistance to Poisoning and Deactivation
High-entropy alloys (HEAs) exhibit superior resistance to poisoning and deactivation compared to platinum in catalytic converters due to their complex multi-element compositions, which create diverse active sites that minimize the adsorption of harmful species like sulfur and carbon monoxide. Platinum catalysts, while highly active, often suffer rapid deactivation when exposed to catalyst poisons, leading to diminished performance over time. The enhanced durability of HEAs under harsh operating conditions makes them promising candidates for long-lasting catalytic converter applications.
Environmental Impact and Sustainability
High-entropy alloys (HEAs) offer significant environmental advantages over traditional platinum catalysts in catalytic converters due to their enhanced durability and reduced reliance on scarce, expensive platinum. The superior oxidation resistance and thermal stability of HEAs contribute to longer catalyst lifespans, decreasing waste generation and resource extraction impacts. Furthermore, HEAs incorporate diverse elemental compositions, enabling sustainable material sourcing and minimizing ecological footprints compared to platinum mining and refining processes.
Scalability and Manufacturing Challenges
High-entropy alloys (HEAs) present scalability challenges due to complex multi-element compositions requiring precise control over phase stability and homogeneity during manufacturing, often demanding advanced synthesis techniques like arc melting or powder metallurgy. Platinum, while expensive, benefits from well-established large-scale manufacturing processes and supply chains, ensuring consistent catalytic performance and cost predictability. The scalability of HEAs for catalytic converters is hindered by difficulties in achieving uniform microstructures and potential material scarcity, whereas platinum catalysts face economic constraints but have mature production infrastructure supporting mass deployment.
Application Case Studies in Automotive Industry
High-entropy alloys (HEAs) demonstrate enhanced catalytic performance and thermal stability compared to traditional platinum catalysts in automotive catalytic converters, particularly in heavy-duty diesel engines. Case studies reveal HEAs sustain higher conversion efficiency for nitrogen oxides and hydrocarbons under fluctuating exhaust conditions, reducing precious metal dependency. This advancement promises cost-effective, durable emissions control solutions aligning with stringent environmental regulations.
Future Perspectives and Technological Advancements
High-entropy alloys (HEAs) offer a promising alternative to platinum in catalytic converters by providing exceptional thermal stability, corrosion resistance, and tunable catalytic properties through multi-element compositions. Advances in nanostructuring and computational design are accelerating the discovery of HEAs with enhanced activity and reduced precious metal content, addressing cost and resource scarcity challenges associated with platinum. Future research aims to optimize HEA surface chemistry and durability under exhaust conditions, potentially revolutionizing catalytic converter efficiency and sustainability in automotive applications.
Infographic: High-entropy alloy vs Platinum for Catalytic converter
 azmater.com
azmater.com