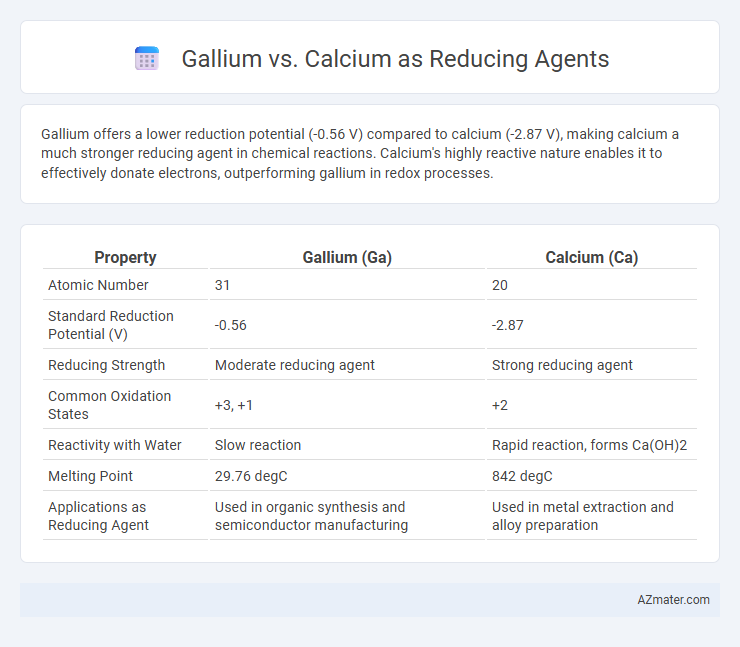
Gallium offers a lower reduction potential (-0.56 V) compared to calcium (-2.87 V), making calcium a much stronger reducing agent in chemical reactions. Calcium's highly reactive nature enables it to effectively donate electrons, outperforming gallium in redox processes.
Table of Comparison
| Property | Gallium (Ga) | Calcium (Ca) |
|---|---|---|
| Atomic Number | 31 | 20 |
| Standard Reduction Potential (V) | -0.56 | -2.87 |
| Reducing Strength | Moderate reducing agent | Strong reducing agent |
| Common Oxidation States | +3, +1 | +2 |
| Reactivity with Water | Slow reaction | Rapid reaction, forms Ca(OH)2 |
| Melting Point | 29.76 degC | 842 degC |
| Applications as Reducing Agent | Used in organic synthesis and semiconductor manufacturing | Used in metal extraction and alloy preparation |
Introduction to Reducing Agents
Reducing agents donate electrons in chemical reactions, facilitating the reduction of other substances. Gallium and calcium serve as effective reducing agents, with calcium exhibiting stronger reducing power due to its lower electronegativity and higher reactivity. Gallium's moderate reducing ability is useful in specific applications where controlled electron transfer is required, contrasting with calcium's more vigorous and less selective action.
Overview of Gallium and Its Properties
Gallium, a post-transition metal with an atomic number of 31, is known for its low melting point of about 29.76degC and remarkable ability to form alloys with various metals. Its high electronegativity and relatively low reduction potential make it an effective reducing agent in chemical reactions, often utilized in organic synthesis and materials science. Compared to calcium, gallium exhibits greater stability in air and moisture, offering advantages in controlled reduction processes while maintaining selective reactivity.
Overview of Calcium and Its Properties
Calcium, an alkaline earth metal with atomic number 20, is a highly reactive metal widely used as a reducing agent in various chemical reactions. Its strong ability to donate electrons stems from its relatively low electronegativity and high electropositivity, making it effective for reducing metal oxides and other compounds. Calcium's abundant availability, moderate melting point of 842degC, and its tendency to form stable ionic compounds contribute to its versatility and efficiency in industrial and laboratory reduction processes.
Chemical Reactivity: Gallium vs Calcium
Gallium exhibits lower chemical reactivity compared to calcium due to its position in the periodic table and electron configuration, making it less prone to losing electrons as a reducing agent. Calcium, a highly reactive alkaline earth metal with a lower ionization energy, acts as a stronger reducing agent by readily donating electrons in redox reactions. The disparity in reactivity arises from calcium's tendency to form ionic bonds easily, while gallium's covalent character and higher electronegativity result in moderated reducing capabilities.
Standard Electrode Potentials Comparison
Gallium exhibits a standard electrode potential of approximately -0.53 V, indicating it is a moderately strong reducing agent but less reactive than calcium, which has a significantly more negative standard electrode potential around -2.87 V. The more negative potential of calcium reflects its greater tendency to lose electrons and reduce other substances compared to gallium. This difference highlights calcium's superior efficacy as a reducing agent in redox reactions where electron donation is critical.
Efficiency of Reduction Reactions
Gallium exhibits higher efficiency as a reducing agent compared to calcium due to its lower standard reduction potential, enabling more controlled and selective electron transfer reactions. Gallium's moderate reactivity allows it to reduce various metal ions effectively without excessive side reactions, whereas calcium's high reactivity often leads to rapid but less controlled reductions. This balance makes gallium preferable in applications requiring precise reduction processes such as in semiconductor manufacturing and organometallic synthesis.
Industrial Applications of Gallium and Calcium
Gallium and calcium serve as reducing agents in various industrial applications, with calcium commonly used for reducing metal oxides in the production of titanium and other refractory metals due to its strong affinity for oxygen. Gallium, while less reactive, is valued in specialized industries for its ability to reduce certain metal ions in electronic materials and semiconductor manufacturing, providing precise control in alloy composition and doping processes. Calcium's cost-effectiveness and high reactivity make it a preferred choice for large-scale metallurgical reductions, whereas gallium's unique properties support niche applications in advanced technology sectors.
Safety Considerations and Handling
Gallium is considered safer than calcium as a reducing agent due to its lower reactivity with air and moisture, minimizing fire and explosion hazards. Handling gallium requires standard laboratory precautions, including gloves and eye protection, while calcium demands more stringent measures because it reacts violently with water and can ignite spontaneously in humid environments. Proper storage of calcium in oil or inert atmospheres is essential, whereas gallium can be stored under ambient conditions with minimal risk.
Cost and Availability Analysis
Gallium's cost significantly exceeds that of calcium, with gallium priced around $300 per kilogram compared to calcium's $2 per kilogram, impacting economic feasibility for large-scale use as a reducing agent. Calcium's abundant availability from natural minerals makes it more accessible and practical for industrial applications, whereas gallium's rarity and complex extraction process restrict supply. Despite gallium's superior chemical properties in some reactions, calcium's lower cost and widespread availability drive preference in many reducing agent applications.
Conclusion: Selecting the Optimal Reducing Agent
Gallium and calcium serve as reducing agents with distinct advantages depending on the application requirements. Calcium offers stronger reducing power and higher reactivity, making it suitable for high-temperature metallurgical processes and deoxidation in steel production. Gallium provides milder reduction conditions and better selectivity, ideal for sensitive chemical syntheses and electronic material fabrication, guiding the selection toward calcium for robust industrial reduction and gallium for precision applications.
Infographic: Gallium vs Calcium for Reducing Agent
 azmater.com
azmater.com