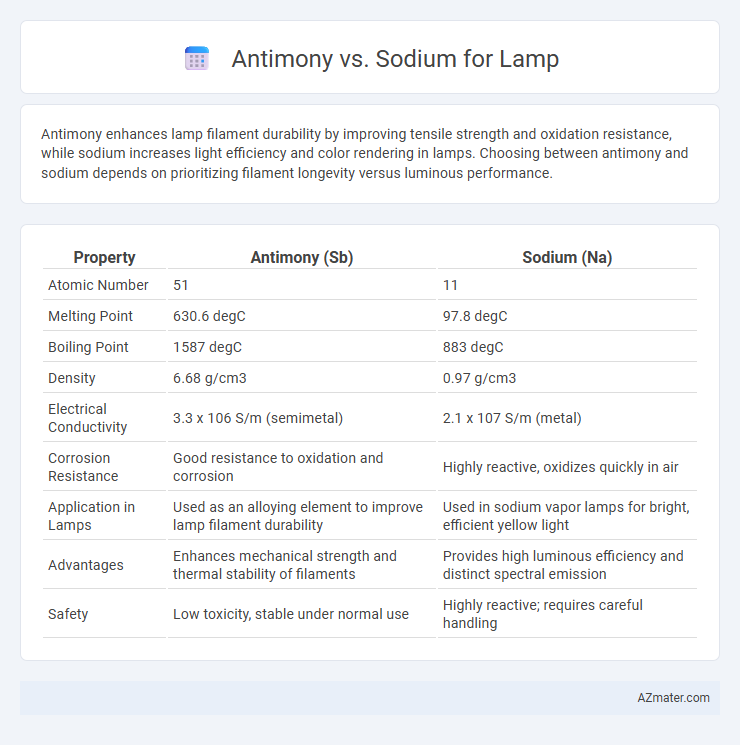
Antimony enhances lamp filament durability by improving tensile strength and oxidation resistance, while sodium increases light efficiency and color rendering in lamps. Choosing between antimony and sodium depends on prioritizing filament longevity versus luminous performance.
Table of Comparison
| Property | Antimony (Sb) | Sodium (Na) |
|---|---|---|
| Atomic Number | 51 | 11 |
| Melting Point | 630.6 degC | 97.8 degC |
| Boiling Point | 1587 degC | 883 degC |
| Density | 6.68 g/cm3 | 0.97 g/cm3 |
| Electrical Conductivity | 3.3 x 106 S/m (semimetal) | 2.1 x 107 S/m (metal) |
| Corrosion Resistance | Good resistance to oxidation and corrosion | Highly reactive, oxidizes quickly in air |
| Application in Lamps | Used as an alloying element to improve lamp filament durability | Used in sodium vapor lamps for bright, efficient yellow light |
| Advantages | Enhances mechanical strength and thermal stability of filaments | Provides high luminous efficiency and distinct spectral emission |
| Safety | Low toxicity, stable under normal use | Highly reactive; requires careful handling |
Introduction to Antimony and Sodium in Lamps
Antimony is primarily used in lamps as an additive to tungsten filaments, improving their strength and resistance to evaporation, thereby enhancing lamp lifespan and performance. Sodium, commonly utilized in high-pressure sodium lamps, produces a distinctive bright yellow light with excellent energy efficiency, making it ideal for street lighting and industrial applications. The distinct chemical properties of antimony and sodium influence lamp design, light quality, and energy consumption, positioning each element for specific lighting technologies.
Chemical Properties and Reactivity
Antimony exhibits moderate reactivity with a melting point of 630.6degC and forms stable compounds such as antimony trioxide (Sb2O3), commonly used in flame retardants, whereas sodium is a highly reactive alkali metal with a low melting point of 97.8degC and readily oxidizes in air, producing sodium oxide. The chemical properties of antimony, including its metalloid nature and resistance to corrosion, contrast sharply with sodium's vigorous reaction with water and propensity to generate intense heat and hydrogen gas. In lamp applications, sodium's high reactivity allows for bright emission spectra ideal for street lighting, while antimony's refractory properties contribute to durability and flame retardancy in lamp components.
Efficiency and Light Output Comparison
Antimony enhances lamp efficiency by improving electrode durability and reducing power consumption, resulting in stable light output with minimal flicker. Sodium lamps deliver higher luminous efficacy, producing intense, warm yellow light with superior brightness for outdoor and industrial applications. Comparing both, sodium lamps excel in light output efficiency, while antimony-added lamps offer improved lifespan and consistent performance under varying electrical conditions.
Color Rendering and Spectrum Quality
Antimony and sodium significantly influence lamp color rendering and spectrum quality, with antimony-doped lamps exhibiting enhanced color rendering index (CRI) due to their broader emission spectrum, which closely resembles natural sunlight. Sodium lamps, especially low-pressure types, emit a narrow spectral line primarily in the yellow region (~589 nm), resulting in poor color rendering and muted color perception. High-pressure sodium lamps provide improved spectrum quality with additional spectral lines but still fall short of the color fidelity achievable with antimony additions.
Lifespan and Maintenance Requirements
Antimony lamps typically offer a longer lifespan compared to sodium lamps due to their enhanced resistance to corrosion and material degradation, resulting in fewer replacements over time. Sodium lamps require more frequent maintenance because their performance diminishes faster under high-temperature conditions and exposure to contaminants, which accelerates gas depletion and electrode wear. The maintenance schedule for antimony lamps is more extended, lowering operational costs while sodium lamps often need regular cleaning and component checks to maintain optimal light output.
Environmental Impact and Safety Concerns
Antimony used in lamps poses significant environmental risks due to its toxicity and persistence, leading to soil and water contamination during disposal and manufacturing. Sodium, while reactive and requiring careful handling to prevent fires or explosions, has a comparatively lower environmental footprint as it breaks down more readily and poses fewer long-term contamination issues. Safety protocols for sodium lamps emphasize containment and inert atmosphere storage, whereas antimony-containing lamps demand stringent hazardous waste management to mitigate ecological damage.
Cost Analysis and Economic Feasibility
Antimony lamps typically exhibit higher initial costs compared to sodium lamps due to the expensive raw materials and complex manufacturing processes involved. Sodium lamps offer superior economic feasibility with lower maintenance expenses and energy-efficient performance, resulting in reduced operational costs over time. When evaluating cost analysis, sodium lamps demonstrate a more attractive return on investment for large-scale lighting projects due to their affordability and longer service life.
Common Applications in Lighting Technology
Antimony is frequently used as a dopant in semiconductor materials for high-intensity discharge (HID) lamps, enhancing electrical conductivity and improving light output efficiency. Sodium, on the other hand, is a key element in sodium vapor lamps, renowned for their high luminous efficacy and warm yellow-orange light, commonly employed in street lighting and industrial applications. Both elements play distinct roles in lighting technology: antimony optimizes performance in HID lamps, while sodium's unique emission spectrum makes it ideal for energy-efficient illumination in outdoor settings.
Advancements and Innovations in Lamp Materials
Antimony enhances lamp efficiency by improving filament strength and oxidation resistance, enabling longer-lasting and more durable lighting elements. Sodium advancements focus on high-pressure sodium lamps, which offer superior luminous efficacy and energy efficiency through innovative gas mixtures and electrode designs. Recent innovations integrate antimony doping with sodium vapor technology to optimize performance, lifespan, and light quality in modern lighting solutions.
Future Trends in Lamp Material Selection
Antimony is gaining attention in lamp material selection due to its superior thermal stability and corrosion resistance, making it suitable for next-generation high-intensity discharge lamps. Sodium remains a popular choice for energy-efficient streetlighting lamps because of its excellent luminous efficacy and low operating temperature. Future trends indicate a shift toward hybrid materials combining antimony and sodium to optimize durability and light quality in advanced lighting systems.
Infographic: Antimony vs Sodium for Lamp
 azmater.com
azmater.com