Copper offers superior electrical conductivity and corrosion resistance compared to zinc, making it ideal for high-performance alloys. Zinc enhances strength and provides cost-effective corrosion protection when combined with copper in brass alloys.
Table of Comparison
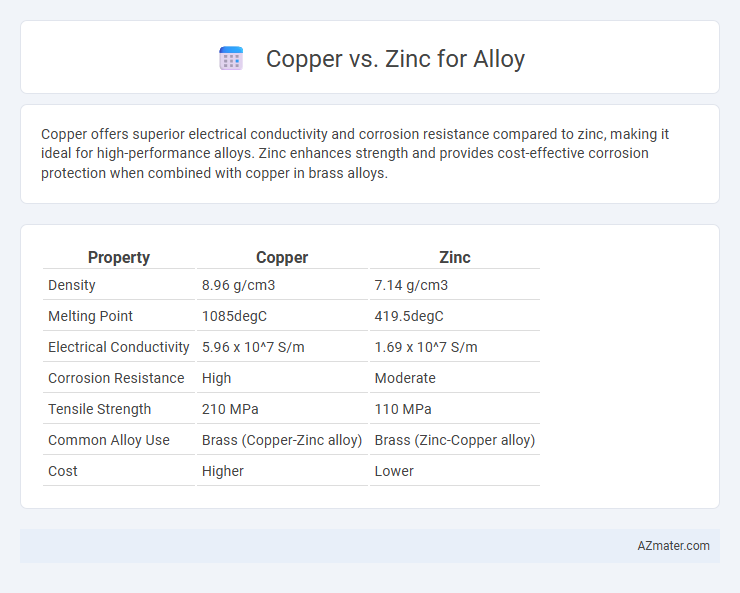
| Property | Copper | Zinc |
|---|---|---|
| Density | 8.96 g/cm3 | 7.14 g/cm3 |
| Melting Point | 1085degC | 419.5degC |
| Electrical Conductivity | 5.96 x 10^7 S/m | 1.69 x 10^7 S/m |
| Corrosion Resistance | High | Moderate |
| Tensile Strength | 210 MPa | 110 MPa |
| Common Alloy Use | Brass (Copper-Zinc alloy) | Brass (Zinc-Copper alloy) |
| Cost | Higher | Lower |
Introduction to Copper and Zinc in Alloys
Copper and zinc are fundamental metals in alloy production, with copper providing excellent electrical conductivity and corrosion resistance, while zinc offers enhanced strength and ductility. Their combination results in brass, a widely used alloy characterized by superior machinability and aesthetic appeal. The proportion of zinc in the copper matrix directly influences the alloy's mechanical properties and corrosion behavior, making copper-zinc alloys versatile for applications in plumbing, musical instruments, and marine equipment.
Physical Properties of Copper vs Zinc
Copper exhibits higher electrical and thermal conductivity compared to zinc, making it ideal for applications requiring efficient energy transfer. Copper has a melting point of 1,085degC and a density of 8.96 g/cm3, whereas zinc melts at 419.5degC with a density of 7.14 g/cm3, indicating zinc's lower thermal stability and lighter weight. The ductility and malleability of copper surpass those of zinc, enabling greater flexibility in alloy formation and mechanical workability.
Chemical Characteristics: Copper vs Zinc
Copper exhibits excellent corrosion resistance and high thermal and electrical conductivity due to its atomic structure, featuring a single 4s electron outside a filled d-subshell. Zinc, with its electron configuration [Ar] 3d10 4s2, offers moderate corrosion resistance and lower conductivity, primarily contributing strength and hardness when alloyed. In alloys like brass, the combination of copper's ductility and zinc's toughness enhances mechanical properties and chemical stability.
Alloying Behavior: Solubility and Compatibility
Copper exhibits excellent solubility with zinc, creating a continuous series of solid solutions that enhance the alloy's strength and corrosion resistance, exemplified by brass. Zinc's compatibility with copper allows for precise control over mechanical properties and color by varying zinc content up to approximately 40%. The mutual solubility and intermetallic phases formed between copper and zinc significantly influence the microstructure and performance of copper-zinc alloys in industrial applications.
Common Copper Alloys and Their Uses
Common copper alloys include brass, an alloy of copper and zinc known for its corrosion resistance and electrical conductivity, widely used in musical instruments and plumbing fittings. Bronze, primarily copper and tin, offers enhanced strength and wear resistance, making it ideal for bearings, ship propellers, and sculptures. Copper-nickel alloys provide excellent resistance to seawater corrosion, often employed in marine hardware and coinage.
Typical Zinc Alloys and Applications
Copper-zinc alloys, commonly known as brass, are widely used due to their excellent corrosion resistance, machinability, and attractive appearance. Typical zinc alloys include alpha brass (up to 35% zinc), which offers high ductility and strength, and cartridge brass (around 30% zinc), favored for its formability in automotive and plumbing applications. These alloys find applications in decorative hardware, electrical connectors, musical instruments, and marine fittings, where a balance of durability and aesthetic appeal is essential.
Mechanical Properties Comparison
Copper exhibits higher ductility and excellent electrical conductivity, making it suitable for applications requiring flexibility and corrosion resistance. Zinc offers superior strength and hardness but lower tensile strength and ductility compared to copper, enhancing wear resistance in alloys. The mechanical properties of copper-zinc alloys vary depending on composition, with brass (copper-zinc alloy) balancing strength and machinability for diverse industrial uses.
Corrosion Resistance: Copper vs Zinc
Copper exhibits superior corrosion resistance compared to zinc, especially in marine and industrial environments due to its ability to form a stable oxide layer that protects the underlying metal. Zinc, while offering sacrificial protection when used as a coating in galvanized steel, tends to corrode more quickly when exposed directly to harsh elements. The longevity and durability of copper alloys make them preferable for applications requiring enhanced resistance to oxidation and environmental degradation.
Cost and Availability Factors
Copper and zinc are essential metals in alloy production, with copper generally being more expensive due to its higher demand and broader industrial applications. Zinc is more abundant and cost-effective, making it a preferred choice for large-scale production of alloys like brass, which balances performance and affordability. The availability of zinc often ensures stable pricing, whereas copper prices can fluctuate significantly depending on global supply and mining conditions.
Choosing the Right Metal for Alloying
Copper offers excellent electrical conductivity and corrosion resistance, making it ideal for alloys used in electrical and marine applications. Zinc enhances strength and hardness in alloys, commonly improving wear resistance in die-casting and brass production. Selecting the right metal depends on balancing properties such as corrosion resistance, mechanical strength, and intended environmental exposure.
Infographic: Copper vs Zinc for Alloy
 azmater.com
azmater.com