Hafnium offers superior corrosion resistance and thermal stability compared to sodium, making it ideal for high-temperature chemical synthesis. Sodium is highly reactive and cost-effective but less stable, limiting its use to low-temperature or less demanding chemical reactions.
Table of Comparison
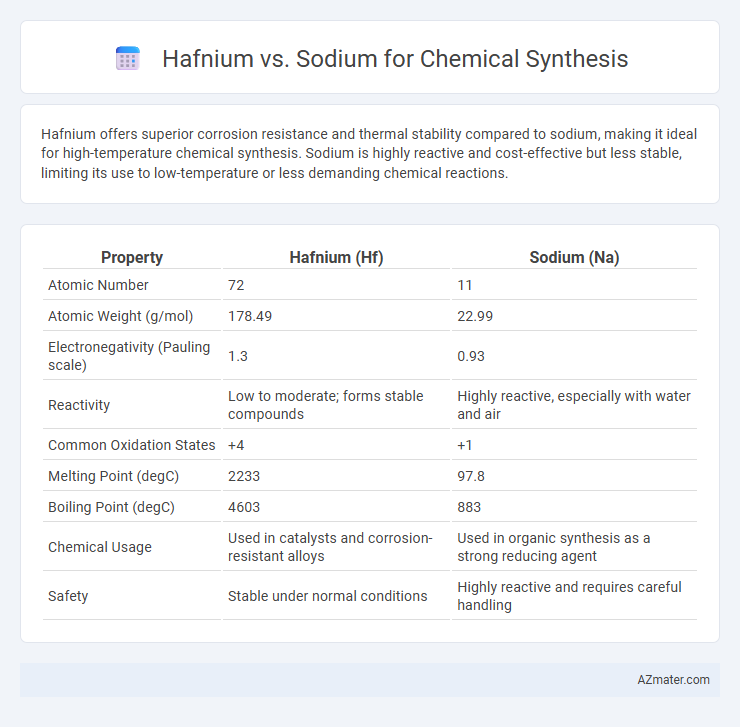
| Property | Hafnium (Hf) | Sodium (Na) |
|---|---|---|
| Atomic Number | 72 | 11 |
| Atomic Weight (g/mol) | 178.49 | 22.99 |
| Electronegativity (Pauling scale) | 1.3 | 0.93 |
| Reactivity | Low to moderate; forms stable compounds | Highly reactive, especially with water and air |
| Common Oxidation States | +4 | +1 |
| Melting Point (degC) | 2233 | 97.8 |
| Boiling Point (degC) | 4603 | 883 |
| Chemical Usage | Used in catalysts and corrosion-resistant alloys | Used in organic synthesis as a strong reducing agent |
| Safety | Stable under normal conditions | Highly reactive and requires careful handling |
Introduction to Hafnium and Sodium
Hafnium (Hf), a transition metal with atomic number 72, is known for its high melting point and excellent corrosion resistance, making it valuable in specialized chemical synthesis applications that require stability under extreme conditions. Sodium (Na), an alkali metal with atomic number 11, exhibits high reactivity, particularly with water and organic compounds, facilitating its widespread use as a reducing agent and in the synthesis of various sodium-containing compounds. The distinct chemical properties of hafnium and sodium influence their roles in synthesis processes, with hafnium often employed for stability and sodium for reactivity.
Chemical Properties: Hafnium vs Sodium
Hafnium exhibits high corrosion resistance and a high melting point of 2233degC, making it ideal for high-temperature chemical synthesis, while sodium, with a low melting point of 98degC, is highly reactive and primarily used as a strong reducing agent in organic synthesis. Hafnium's chemical inertness and ability to form stable compounds contrast with sodium's aggressive reactivity, which requires careful handling under inert atmospheres. The differences in electronegativity and oxidation states--Hafnium commonly in +4, Sodium in +1--greatly influence their roles and compatibility in diverse chemical reactions.
Reactivity Comparison in Synthesis
Hafnium exhibits lower reactivity compared to sodium, making it more suitable for controlled chemical synthesis involving sensitive substrates. Sodium's high reactivity and strong reducing properties enable rapid electron transfer, driving vigorous reactions often used in organic reductions and metal alloy formation. The choice between hafnium and sodium depends on reaction conditions, desired selectivity, and the stability of intermediate species during synthesis processes.
Role in Catalysis and Reaction Mechanisms
Hafnium serves as an effective catalyst in various chemical syntheses due to its ability to facilitate complex reaction mechanisms, especially in polymerization and hydrogenation processes, by stabilizing reactive intermediates through its high Lewis acidity. Sodium, while less commonly used directly as a catalyst, often acts as a strong reducing agent in synthesis and can influence reaction pathways by altering electron transfer steps and promoting nucleophilic substitutions. The distinct electronic configurations of hafnium and sodium result in fundamentally different catalytic behaviors, impacting reaction rates, selectivity, and the formation of target compounds in practical applications.
Common Uses in Organic Synthesis
Hafnium serves as a robust catalyst in organic synthesis, particularly in hydrocarbon upgrading and selective hydrogenation reactions due to its high melting point and strong Lewis acidity. Sodium, commonly used as a reducing agent, is essential for reactions like the Birch reduction and deprotonation of active methylene compounds, enabling key transformations in organic synthesis. Both elements play distinct roles, with hafnium facilitating catalytic processes while sodium drives reduction and base-mediated reactions.
Safety Considerations and Handling
Hafnium offers enhanced safety in chemical synthesis due to its lower reactivity compared to sodium, minimizing risks of violent reactions or explosions. Sodium's highly reactive nature with moisture demands stringent handling protocols, including inert atmosphere storage and strict exclusion of water to prevent hazardous fires. Proper personal protective equipment and specialized training are essential when working with sodium to mitigate risks, whereas hafnium's stability allows safer manipulation under less restrictive conditions.
Availability and Cost Analysis
Hafnium, a rare transition metal primarily obtained as a byproduct of zirconium refining, has limited availability, leading to higher market prices compared to sodium, which is abundant and inexpensive, derived mainly from common salt deposits. Sodium's widespread availability and low extraction cost make it a preferred reducing agent in large-scale chemical synthesis, whereas hafnium's high cost restricts its use to specialized applications requiring specific properties like high melting point and corrosion resistance. The economic feasibility of chemical synthesis favors sodium due to its cost-effectiveness and accessibility, while hafnium remains niche due to resource scarcity and processing expenses.
Environmental Impact and Sustainability
Hafnium and sodium differ significantly in environmental impact and sustainability within chemical synthesis; hafnium, a rare transition metal, has limited availability and involves energy-intensive extraction processes, raising concerns about resource depletion and ecological disturbance. Sodium, abundant and derived primarily from salt, offers a more sustainable option due to its lower environmental footprint and recyclability in synthesis reactions. The choice between hafnium and sodium depends on balancing reactivity needs with the pursuit of greener chemistry and long-term resource management.
Case Studies: Hafnium and Sodium in Synthesis
Case studies highlight hafnium's role as a robust catalyst in olefin polymerization, enabling high activity and selectivity due to its variable oxidation states and strong Lewis acidity. Sodium excels in organic synthesis as a powerful reducing agent, frequently employed in Birch reductions and reductive couplings, favored for its affordability and reactivity. Comparative analysis shows hafnium offers precision in catalytic cycles for complex molecular architectures, while sodium provides cost-effective, large-scale applicability in reductive transformations.
Choosing the Right Metal for Your Application
Hafnium offers superior corrosion resistance, high melting point, and excellent mechanical stability, making it ideal for high-temperature and aerospace chemical synthesis applications. Sodium, with its high reactivity and lower cost, is preferred in organic reductions and large-scale industrial processes where rapid reactions are advantageous. Selecting the right metal depends on reaction conditions, desired product purity, and cost-efficiency requirements.
Infographic: Hafnium vs Sodium for Chemical Synthesis
 azmater.com
azmater.com