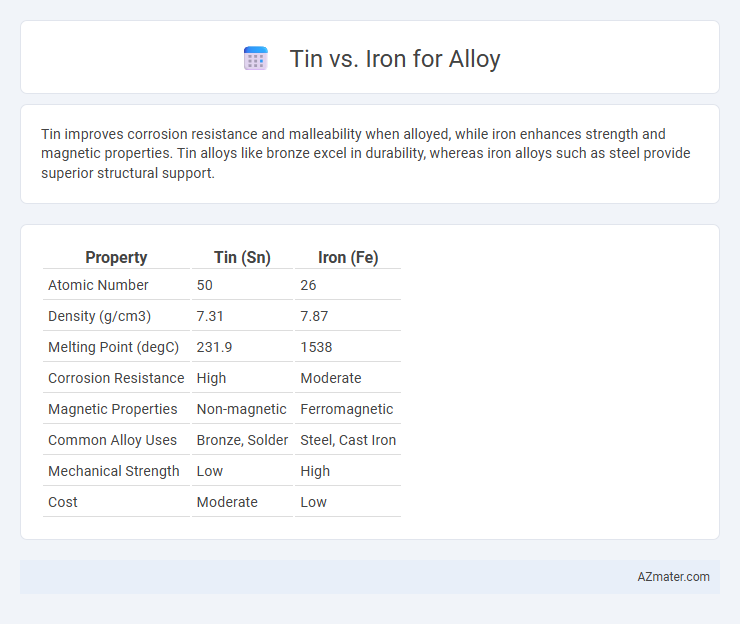
Tin improves corrosion resistance and malleability when alloyed, while iron enhances strength and magnetic properties. Tin alloys like bronze excel in durability, whereas iron alloys such as steel provide superior structural support.
Table of Comparison
| Property | Tin (Sn) | Iron (Fe) |
|---|---|---|
| Atomic Number | 50 | 26 |
| Density (g/cm3) | 7.31 | 7.87 |
| Melting Point (degC) | 231.9 | 1538 |
| Corrosion Resistance | High | Moderate |
| Magnetic Properties | Non-magnetic | Ferromagnetic |
| Common Alloy Uses | Bronze, Solder | Steel, Cast Iron |
| Mechanical Strength | Low | High |
| Cost | Moderate | Low |
Introduction to Tin and Iron as Alloying Elements
Tin and iron are essential alloying elements used to enhance metal properties for various industrial applications. Tin, primarily added to copper to produce bronze, improves corrosion resistance, strength, and machinability, making it ideal for marine and decorative uses. Iron, alloyed with carbon to form steel, increases hardness, tensile strength, and durability, serving as the backbone of construction, automotive, and manufacturing industries.
Physical Properties: Tin vs Iron
Tin has a low melting point of 232degC and a density of 7.31 g/cm3, making it lightweight and easy to mold, while iron boasts a much higher melting point of 1538degC and a density of 7.87 g/cm3, resulting in greater strength and durability. Tin exhibits excellent corrosion resistance and a relatively soft, malleable structure, in contrast to iron's hardness and magnetic properties. These differences in physical properties influence their specific applications in alloy production, with tin often used to improve corrosion resistance and castability, and iron contributing hardness and structural integrity.
Chemical Properties and Reactivity
Tin exhibits low reactivity, demonstrating resistance to corrosion and oxidation, which stabilizes alloys by preventing rapid degradation. Iron, with higher chemical reactivity, readily reacts with oxygen and moisture, forming oxides that can weaken alloy structures unless alloyed with protective elements. Combining tin's corrosion resistance with iron's strength enhances alloy durability and mechanical performance in various industrial applications.
Historical Uses in Alloy Formation
Tin and iron have historically played crucial roles in alloy formation, particularly in the development of bronze and steel. Bronze, an alloy of copper and tin, was a breakthrough material during the Bronze Age, enhancing tool durability and weapon strength, whereas iron's prominence rose in the Iron Age due to its abundance and superior tensile properties. Early iron alloys, often combined with small amounts of carbon and other elements, led to the creation of steel, which revolutionized construction, warfare, and agriculture with its enhanced hardness and flexibility.
Role of Tin in Common Alloys
Tin enhances alloy corrosion resistance and improves workability, making it essential in bronze and solder compositions. Its presence in alloys reduces brittleness, providing durability in applications ranging from marine hardware to electrical components. Tin's ability to form stable intermetallic compounds with metals like copper underpins its vital role in improving alloy strength and longevity.
Role of Iron in Common Alloys
Iron plays a crucial role in common alloys such as steel, where it serves as the primary base metal, providing strength, toughness, and magnetic properties. Unlike tin, which is mainly used in alloys like bronze to improve corrosion resistance and machinability, iron's versatility enables the formation of a wide range of alloys with varying carbon content and other elements to enhance hardness, ductility, and wear resistance. The presence of iron in alloys significantly impacts mechanical performance and applications in construction, automotive, and machinery industries.
Mechanical Strength Comparison
Tin alloys typically exhibit lower mechanical strength compared to iron alloys, as iron's atomic structure provides higher tensile strength and hardness essential for load-bearing applications. Iron alloys, particularly steel, demonstrate superior yield strength and fatigue resistance, making them ideal for structural components requiring durability. While tin can improve malleability and corrosion resistance, it does not enhance the fundamental mechanical strength to the level that iron alloys achieve.
Corrosion Resistance: Tin vs Iron
Tin offers superior corrosion resistance compared to iron due to its ability to form a stable, protective oxide layer that prevents further oxidation. Iron, prone to rust when exposed to moisture and oxygen, rapidly degrades without protective coatings or alloying elements. The incorporation of tin in alloys significantly enhances longevity and durability in corrosive environments by reducing oxidation rates and preventing structural damage.
Cost and Availability in Alloy Production
Tin is generally more expensive and less abundant than iron, impacting alloy production costs significantly. Iron's widespread availability and lower price make it the preferred choice for large-scale alloy manufacturing. Cost efficiency in alloy production often favors iron-based alloys due to tin's limited supply and higher market value.
Applications and Industry Preferences
Tin is extensively used in alloys such as bronze for corrosion resistance and electrical conductivity, making it ideal for electronics, automotive, and packaging industries. Iron-based alloys like steel dominate construction, machinery, and transportation due to superior strength and versatility. Industrial preferences favor tin alloys for lightweight, anti-corrosive applications, while iron alloys are preferred where structural integrity and durability are critical.
Infographic: Tin vs Iron for Alloy
 azmater.com
azmater.com