High-entropy alloys exhibit superior structural stability and corrosion resistance compared to nickel, enhancing battery lifespan and performance. Their complex atomic composition improves electrochemical properties, offering higher capacity and cycling stability in battery applications.
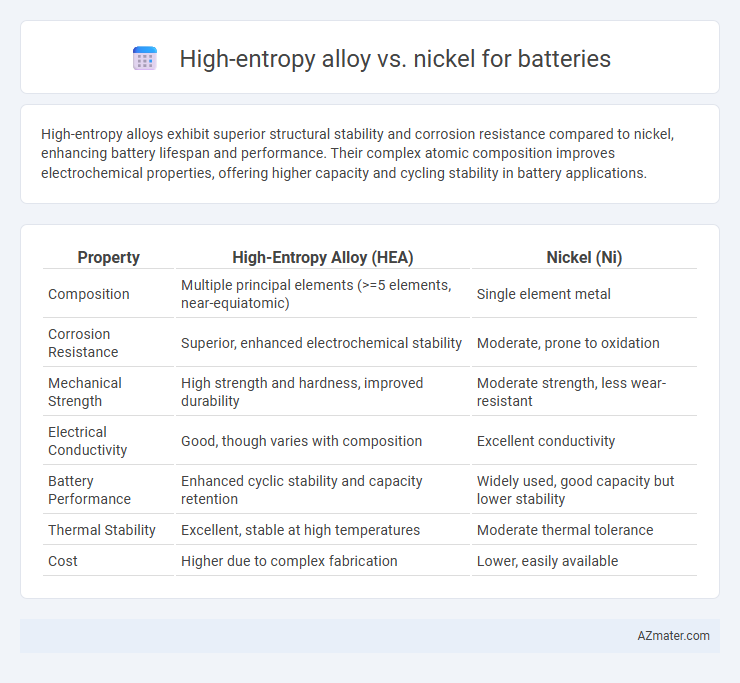
Table of Comparison
| Property | High-Entropy Alloy (HEA) | Nickel (Ni) |
|---|---|---|
| Composition | Multiple principal elements (>=5 elements, near-equiatomic) | Single element metal |
| Corrosion Resistance | Superior, enhanced electrochemical stability | Moderate, prone to oxidation |
| Mechanical Strength | High strength and hardness, improved durability | Moderate strength, less wear-resistant |
| Electrical Conductivity | Good, though varies with composition | Excellent conductivity |
| Battery Performance | Enhanced cyclic stability and capacity retention | Widely used, good capacity but lower stability |
| Thermal Stability | Excellent, stable at high temperatures | Moderate thermal tolerance |
| Cost | Higher due to complex fabrication | Lower, easily available |
Introduction to High-Entropy Alloys and Nickel in Batteries
High-entropy alloys (HEAs) consist of multiple principal elements in near-equiatomic ratios, offering exceptional mechanical stability, corrosion resistance, and thermal stability, making them promising candidates for advanced battery electrodes. Nickel, a widely used transition metal in batteries, demonstrates excellent electrochemical performance and conductivity but faces challenges like capacity fading and structural degradation. Comparing HEAs to nickel highlights the potential for HEA materials to overcome conventional limitations, enhancing battery lifespan and efficiency through their unique multi-element synergy and configurational entropy effects.
Composition and Structure: High-Entropy Alloys vs Nickel
High-entropy alloys (HEAs) comprise multiple principal elements, often five or more in near-equiatomic ratios, creating a complex, multi-component matrix with high configurational entropy stabilizing a single-phase solid solution. In contrast, nickel, a single-element metal, offers a simpler face-centered cubic (FCC) crystal structure with well-known electrochemical properties. The multi-element composition of HEAs provides enhanced structural stability, corrosion resistance, and tunable electronic properties, making them promising candidates for advanced battery electrodes compared to traditional nickel-based materials.
Electrochemical Properties Comparison
High-entropy alloys (HEAs) exhibit enhanced electrochemical stability and higher corrosion resistance compared to conventional nickel electrodes in battery applications, leading to improved cycle life and capacity retention. The multi-element composition of HEAs facilitates superior charge transfer kinetics and reduced electrode polarization, resulting in increased energy efficiency. In contrast, nickel electrodes often suffer from structural degradation and capacity fading due to limited electrochemical stability under prolonged cycling conditions.
Energy Density and Capacity Analysis
High-entropy alloys (HEAs) exhibit superior energy density and capacity retention compared to conventional nickel electrodes in battery applications, attributed to their multi-element composition that enhances structural stability and electrochemical performance. HEAs demonstrate improved cycle life and reduced capacity fading by mitigating common degradation mechanisms such as volume expansion and electrode pulverization. This results in higher specific capacity and more efficient charge-discharge cycles, making HEAs a promising alternative to nickel for advanced battery technologies.
Cycle Life and Durability of Both Materials
High-entropy alloys exhibit superior cycle life and durability compared to traditional nickel-based materials due to their multi-element composition that enhances structural stability and resistance to degradation during repeated charge-discharge cycles. Nickel, while widely used in battery cathodes for its high energy density, tends to suffer from capacity fading and structural fatigue over extended cycling. The unique lattice distortion in high-entropy alloys effectively mitigates mechanical stress and corrosion, leading to prolonged battery lifespan and improved performance retention.
Cost and Material Availability
High-entropy alloys (HEAs) offer a promising alternative to nickel in battery applications due to their diverse elemental composition, which can reduce dependence on costly and supply-restricted nickel. While nickel prices have experienced volatility driven by geopolitical factors and increasing demand in lithium-ion batteries, HEAs leverage more abundant elements, enhancing material availability and potentially lowering overall costs. Production challenges and scalability of HEAs remain factors, but their ability to integrate multiple inexpensive metals positions them as a cost-effective and sustainable option compared to nickel-centric battery materials.
Safety and Thermal Stability Considerations
High-entropy alloys exhibit superior thermal stability compared to nickel, reducing risks of thermal runaway in battery applications due to their complex multi-element composition and robust phase structures. Their enhanced safety profiles arise from improved oxidation resistance and structural integrity at elevated temperatures, crucial for maintaining battery performance under thermal stress. Nickel, while providing high energy density, tends to suffer from thermal instability and increased hazard potential during overheating scenarios in batteries.
Recent Innovations and Research Developments
Recent innovations in high-entropy alloys (HEAs) for battery applications demonstrate their superior structural stability and enhanced electrochemical performance compared to conventional nickel-based materials. Research developments highlight HEAs' multi-element compositions that improve resistance to corrosion, reduce volumetric changes during charge-discharge cycles, and enable higher capacity retention. These advancements position HEAs as promising alternatives to nickel in developing next-generation batteries with improved lifespan and energy density.
Environmental Impact and Sustainability
High-entropy alloys (HEAs) in batteries offer enhanced durability and corrosion resistance, leading to longer battery lifespans and reduced waste compared to traditional nickel-based electrodes. Nickel extraction involves intensive mining processes that contribute to significant environmental degradation and high carbon emissions, while HEAs often incorporate more abundant and less environmentally damaging elements. Sustainable battery technologies increasingly favor HEAs due to their potential to lower resource depletion and minimize ecological footprints throughout the battery lifecycle.
Future Prospects and Industry Adoption
High-entropy alloys (HEAs) offer superior structural stability and corrosion resistance compared to conventional nickel-based materials, making them promising candidates for next-generation battery electrodes. Their unique multi-element composition enhances electrochemical performance and cycle life, positioning HEAs as key materials for future energy storage solutions in electric vehicles and grid storage. Industry adoption is accelerating as research demonstrates HEAs' potential to overcome limitations of nickel, driving investment in scalable manufacturing technologies for commercial battery applications.
Infographic: High-entropy alloy vs Nickel for Battery
 azmater.com
azmater.com