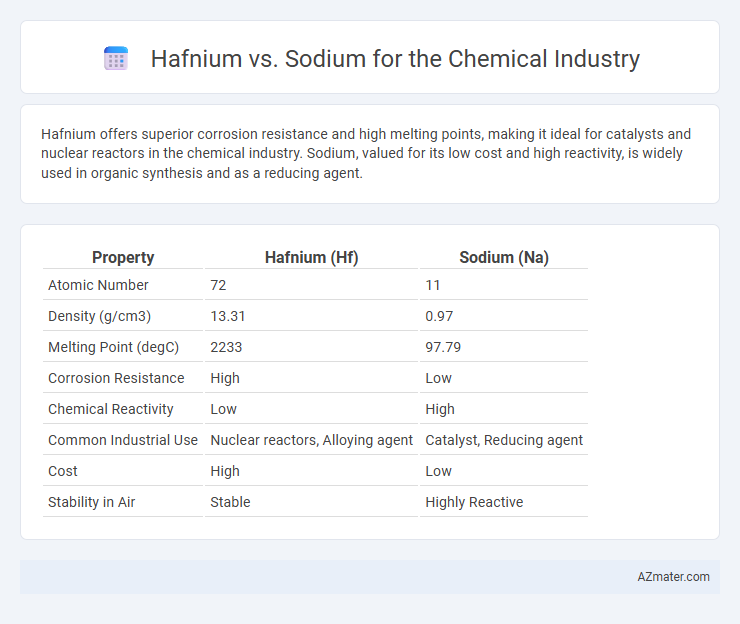
Hafnium offers superior corrosion resistance and high melting points, making it ideal for catalysts and nuclear reactors in the chemical industry. Sodium, valued for its low cost and high reactivity, is widely used in organic synthesis and as a reducing agent.
Table of Comparison
| Property | Hafnium (Hf) | Sodium (Na) |
|---|---|---|
| Atomic Number | 72 | 11 |
| Density (g/cm3) | 13.31 | 0.97 |
| Melting Point (degC) | 2233 | 97.79 |
| Corrosion Resistance | High | Low |
| Chemical Reactivity | Low | High |
| Common Industrial Use | Nuclear reactors, Alloying agent | Catalyst, Reducing agent |
| Cost | High | Low |
| Stability in Air | Stable | Highly Reactive |
Introduction to Hafnium and Sodium in the Chemical Industry
Hafnium, a lustrous, ductile transition metal, plays a critical role in the chemical industry due to its high corrosion resistance and neutron absorption properties, making it essential for nuclear reactor control rods and specialized corrosion-resistant alloys. Sodium, an alkali metal, is widely used for its excellent reactivity and reducing properties, crucial in the production of chemicals like sodium hydroxide, sodium vapor lamps, and organic synthesis. Both elements offer unique advantages; hafnium provides high durability in extreme environments, while sodium delivers versatile applications in large-scale industrial chemical processes.
Elemental Properties: Hafnium vs Sodium
Hafnium exhibits high melting point (2233degC), excellent corrosion resistance, and strong neutron absorption, making it valuable in nuclear reactors and high-temperature applications. Sodium, with a low melting point (98degC) and high reactivity, is primarily used as a reducing agent and in organic synthesis. The elemental density difference (hafnium: 13.31 g/cm3 vs sodium: 0.97 g/cm3) significantly influences their industrial applications, with hafnium favored for durability and stability, while sodium's reactivity enables versatile chemical processes.
Availability and Supply Chain Considerations
Hafnium is a rare transition metal primarily obtained as a byproduct of zirconium refining, leading to limited availability and a complex supply chain heavily reliant on specialized extraction processes. Sodium, by contrast, is plentiful and easily sourced from abundant natural resources such as salt and brine, resulting in a robust and well-established supply chain with lower production costs. The restricted global production capacity of hafnium versus the widespread distribution and accessibility of sodium significantly impact their respective feasibility and scalability in chemical industry applications.
Chemical Reactivity and Stability Comparison
Hafnium exhibits exceptional chemical stability with resistance to corrosion and oxidation, making it ideal for high-temperature applications in the chemical industry. Sodium, characterized by its high reactivity, aggressively reacts with water and air, posing handling challenges but serving as a potent reducing agent in synthesis processes. The stark contrast between hafnium's inertness and sodium's vigorous reactivity defines their distinct roles in industrial chemical reactions and material selection.
Industrial Applications of Hafnium
Hafnium is extensively used in the chemical industry for its exceptional corrosion resistance and high melting point, making it ideal for manufacturing reactor control rods and highly durable alloys in nuclear reactors. Sodium, while useful as a coolant in certain nuclear reactors, lacks the versatility and stability of hafnium under extreme chemical conditions. Hafnium's unique ability to absorb neutrons efficiently also positions it as a critical material for enhancing safety and performance in chemical processing and nuclear energy applications.
Industrial Applications of Sodium
Sodium plays a crucial role in the chemical industry due to its widespread use as a reducing agent and catalyst in organic synthesis and industrial processes such as the production of synthetic rubber and detergents. Its highly reactive nature enables efficient processing in the manufacture of chemicals like sodium hydride, sodium borohydride, and sodium cyanide, which are essential for pharmaceuticals and agrochemicals. Unlike hafnium, which is primarily used in nuclear reactors and aerospace alloys, sodium's versatility and lower cost make it indispensable in large-scale chemical manufacturing.
Safety and Handling Differences
Hafnium, a heavy transition metal, requires strict safety protocols due to its high melting point and tendency to form hazardous oxides, whereas sodium, a highly reactive alkali metal, demands careful handling under inert atmospheres to prevent violent reactions with water and air. Sodium's reactivity necessitates storage in mineral oil or sealed containers, while hafnium's relative stability reduces risks of spontaneous ignition but requires precautions against dust inhalation. Industrial processes involving sodium focus on controlling exothermic reactions, whereas hafnium handling emphasizes protection from particulate contamination and elevated temperatures.
Environmental Impact and Sustainability
Hafnium offers advantages in chemical industries due to its corrosion resistance and stability, reducing hazardous waste generation compared to sodium, which is highly reactive and poses significant handling risks and environmental hazards. Sodium production often relies on energy-intensive processes leading to higher carbon footprints, while hafnium's applications, despite higher extraction energy, benefit from longer lifecycle and recyclability, promoting sustainability. The environmental impact of sodium's reactive nature demands strict containment measures to prevent soil and water contamination, contrasting with hafnium's more inert profile that supports eco-friendly industrial practices.
Cost Analysis: Hafnium vs Sodium
Hafnium's high cost, driven by its rarity and complex extraction process, contrasts sharply with sodium's affordability due to abundant availability and straightforward production. Industrial applications requiring corrosion resistance or neutron absorption justify hafnium's premium pricing despite economic challenges. Conversely, sodium's low cost and widespread use in large-scale chemical processes make it the preferred choice when budget constraints and scalability dominate.
Future Trends and Innovations in Chemical Industry Uses
Hafnium's unique properties, such as its high melting point and corrosion resistance, position it as a critical material for advanced reactors and semiconductor manufacturing, driving innovation in high-performance chemical processes. Sodium's abundance and cost-effectiveness continue to make it indispensable in large-scale chemical production, particularly in organic synthesis and as a reducing agent. Future trends indicate increased integration of hafnium in niche, high-tech applications, while sodium remains essential for sustainable industrial chemistry due to its versatility and availability.
Infographic: Hafnium vs Sodium for Chemical Industry
 azmater.com
azmater.com