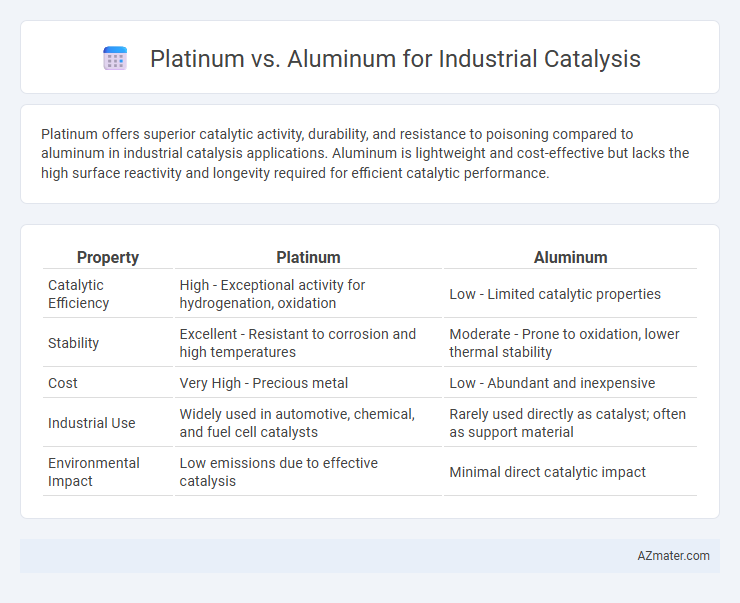
Platinum offers superior catalytic activity, durability, and resistance to poisoning compared to aluminum in industrial catalysis applications. Aluminum is lightweight and cost-effective but lacks the high surface reactivity and longevity required for efficient catalytic performance.
Table of Comparison
| Property | Platinum | Aluminum |
|---|---|---|
| Catalytic Efficiency | High - Exceptional activity for hydrogenation, oxidation | Low - Limited catalytic properties |
| Stability | Excellent - Resistant to corrosion and high temperatures | Moderate - Prone to oxidation, lower thermal stability |
| Cost | Very High - Precious metal | Low - Abundant and inexpensive |
| Industrial Use | Widely used in automotive, chemical, and fuel cell catalysts | Rarely used directly as catalyst; often as support material |
| Environmental Impact | Low emissions due to effective catalysis | Minimal direct catalytic impact |
Introduction to Industrial Catalysts
Platinum and aluminum serve distinct roles in industrial catalysis, with platinum commonly used as an active catalytic metal due to its excellent chemical stability and high catalytic activity in reactions like hydrogenation and oxidation. Aluminum, primarily in the form of alumina (Al2O3), functions as a crucial catalyst support material that provides high surface area, thermal stability, and mechanical strength to enhance catalyst dispersion and performance. The interplay between platinum as the active catalyst and aluminum-based supports is essential for optimizing reaction efficiency, selectivity, and catalyst durability in various industrial processes.
Overview of Platinum as a Catalyst
Platinum is a highly efficient catalyst known for its exceptional ability to facilitate hydrogenation, oxidation, and reforming reactions in industrial processes. Its superior catalytic activity and resistance to high temperatures make it ideal for applications in automotive catalytic converters and petroleum refining. Although more expensive than aluminum-based catalysts, platinum's durability and selectivity significantly enhance reaction rates and product yield.
Overview of Aluminum as a Catalyst
Aluminum serves as a valuable catalyst support in industrial catalysis due to its high surface area, thermal stability, and cost-effectiveness compared to platinum. Its abundant availability and strong mechanical properties make aluminum oxides ideal for dispersing active catalytic metals, enhancing reaction rates and selectivity in processes such as hydrocarbon reforming and oxidation. Aluminum's versatility and resistance to sintering contribute to sustained catalytic performance under harsh industrial conditions.
Mechanisms of Catalytic Action: Platinum vs Aluminum
Platinum exhibits superior catalytic activity in industrial processes due to its ability to facilitate electron transfer and adsorb reactants via d-orbital interactions, enabling mechanisms such as hydrogenation and oxidation with high efficiency. Aluminum, primarily used as a support material or promoter, contributes by providing a high-surface-area oxide framework that enhances catalyst dispersion and stability but lacks intrinsic catalytic capabilities. The fundamental difference lies in platinum's active role in bond activation through its variable oxidation states, whereas aluminum serves indirectly by modifying the catalyst environment to optimize reaction conditions.
Efficiency and Performance Comparison
Platinum catalysts exhibit superior catalytic efficiency and stability in industrial reactions such as hydrogenation and oxidation, outperforming aluminum-based catalysts which are typically less active and prone to deactivation. The high surface area and electronic properties of platinum enhance reaction rates and selectivity, making it ideal for processes requiring precise control and high conversion rates. Aluminum, often used as a support material rather than a primary catalyst, contributes to structural integrity but lacks the intrinsic catalytic activity that defines platinum's performance in industrial applications.
Cost Analysis: Platinum and Aluminum Catalysts
Platinum catalysts offer superior catalytic activity and durability but come with significantly higher costs due to their scarcity and complex extraction process. Aluminum catalysts, primarily used as supports or in zeolite forms, provide a cost-effective alternative with lower performance metrics, making them suitable for large-scale, budget-constrained industrial applications. The cost analysis reveals that while platinum ensures higher efficiency and longer lifespan, aluminum-based catalysts reduce overall production expenses, balancing catalytic effectiveness with financial feasibility.
Durability and Lifespan in Industrial Settings
Platinum exhibits superior durability and a significantly longer lifespan than aluminum in industrial catalysis due to its high resistance to oxidation, corrosion, and thermal degradation. While aluminum can suffer from rapid wear and corrosion under harsh catalytic conditions, platinum maintains catalytic activity and structural integrity over extended operation periods, reducing maintenance downtime. These properties make platinum the preferred choice in high-performance applications where catalyst longevity and consistent efficiency are critical.
Environmental Impact and Sustainability
Platinum demonstrates superior catalytic efficiency with minimal emissions but involves high environmental costs due to mining and resource scarcity. Aluminum offers a more abundant and recyclable option, reducing environmental footprint despite lower catalytic activity in certain reactions. Sustainable industrial catalysis increasingly favors aluminum-based catalysts to balance performance with eco-friendly practices and resource conservation.
Applications and Industry Use Cases
Platinum is extensively used in catalytic converters for automotive exhaust systems, fuel cells, and chemical synthesis due to its superior catalytic activity and resistance to poisoning. Aluminum, often employed as alumina supports, enhances catalyst stability and surface area in petrochemical refining and environmental cleanup processes. The synergy of platinum's catalytic properties with aluminum's structural benefits is critical in industries such as oil refining, pharmaceutical production, and renewable energy technologies.
Future Trends in Catalyst Development
Platinum remains a benchmark metal in industrial catalysis due to its exceptional activity and selectivity, yet its high cost and scarcity drive research toward aluminum-based catalysts, which offer abundant supply and lightweight advantages. Emerging trends emphasize the development of platinum-aluminum hybrid catalysts and nanostructured materials to enhance durability, reduce platinum loading, and improve catalytic efficiency for sustainable industrial applications. Advances in computational modeling and machine learning accelerate the design of tailored catalysts, optimizing metal-support interactions and electronic properties to meet future demands in energy conversion and chemical synthesis.
Infographic: Platinum vs Aluminum for Industrial Catalysis
 azmater.com
azmater.com