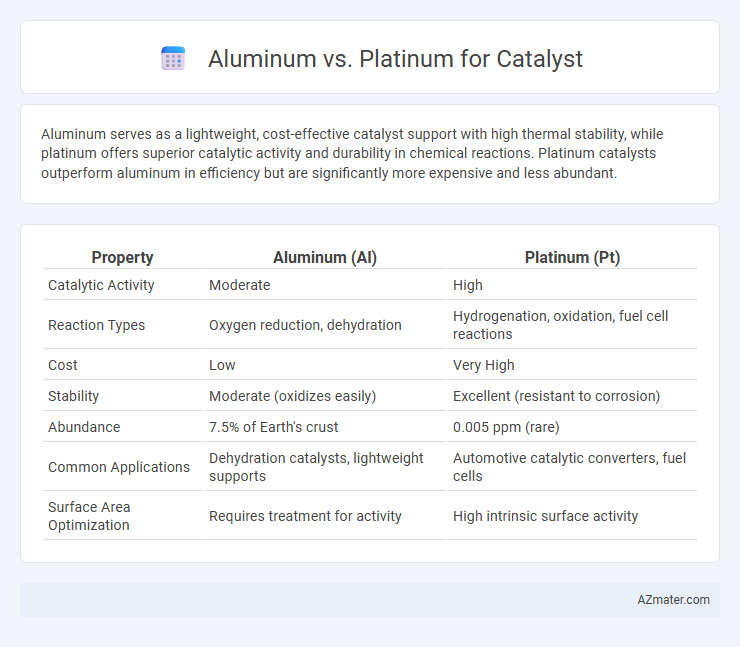
Aluminum serves as a lightweight, cost-effective catalyst support with high thermal stability, while platinum offers superior catalytic activity and durability in chemical reactions. Platinum catalysts outperform aluminum in efficiency but are significantly more expensive and less abundant.
Table of Comparison
| Property | Aluminum (Al) | Platinum (Pt) |
|---|---|---|
| Catalytic Activity | Moderate | High |
| Reaction Types | Oxygen reduction, dehydration | Hydrogenation, oxidation, fuel cell reactions |
| Cost | Low | Very High |
| Stability | Moderate (oxidizes easily) | Excellent (resistant to corrosion) |
| Abundance | 7.5% of Earth's crust | 0.005 ppm (rare) |
| Common Applications | Dehydration catalysts, lightweight supports | Automotive catalytic converters, fuel cells |
| Surface Area Optimization | Requires treatment for activity | High intrinsic surface activity |
Introduction to Catalysts: Aluminum and Platinum
Aluminum and platinum serve distinct roles as catalysts in chemical reactions due to their unique properties. Aluminum, often used as aluminum oxide, acts as a catalyst support or a mild catalyst in processes like the production of synthetic fuels and petrochemical cracking. Platinum offers superior catalytic activity and stability in applications such as automotive catalytic converters and hydrogen fuel cells, enabling efficient oxidation and reduction reactions.
Chemical Properties and Reactivity
Aluminum, characterized by its high affinity for oxygen and moderate catalytic activity, serves primarily as a support material in catalytic reactions due to its amphoteric oxide layer, which facilitates adsorption processes. Platinum exhibits superior catalytic properties owing to its d-electron configuration, enabling efficient adsorption and activation of molecules such as hydrogen and oxygen, crucial for catalytic converters and hydrogenation reactions. The chemical stability and resistance to oxidation of platinum contrast with aluminum's susceptibility to forming a passive oxide film, impacting their reactivity and durability in catalytic applications.
Catalytic Efficiency: Aluminum vs Platinum
Platinum exhibits significantly higher catalytic efficiency than aluminum due to its superior ability to adsorb and activate reactant molecules on its surface, facilitating faster reaction rates in processes like hydrogenation and oxidation. Aluminum, while abundant and cost-effective, demonstrates lower catalytic performance primarily because of its limited electronic properties and less effective surface interactions with reactants. Industrial applications consistently favor platinum catalysts for reactions requiring high turnover frequency and selectivity, despite the metal's higher cost and scarcity.
Common Industrial Applications
Aluminum oxide is widely used as a catalyst support in industrial processes such as fluid catalytic cracking (FCC) in petroleum refining due to its high surface area and thermal stability. Platinum catalysts are essential in automotive catalytic converters and chemical synthesis, including hydrogenation reactions, because of their excellent catalytic activity and resistance to poisoning. Both metals serve critical roles: aluminum oxide enhances catalyst dispersion, while platinum provides superior catalytic performance in oxidation and reduction reactions.
Cost and Economic Considerations
Aluminum catalysts offer significantly lower costs compared to platinum, making them more economically viable for large-scale industrial applications. Platinum catalysts, while highly effective and durable, come with high market prices and limited availability, impacting overall process cost-efficiency. The choice between aluminum and platinum catalysts requires balancing initial investment against catalytic performance and lifecycle expenses.
Environmental Impact and Sustainability
Aluminum catalysts, especially in their oxide form, offer a lower environmental footprint due to their abundance, recyclability, and low toxicity, making them more sustainable choices for industrial applications. Platinum catalysts, despite their high efficiency and selectivity in chemical reactions, contribute to environmental concerns due to resource scarcity, high energy demand in extraction, and difficulties in recycling. Sustainable catalyst development increasingly favors aluminum-based materials to reduce ecological impact while maintaining catalytic performance.
Durability and Lifespan as Catalysts
Platinum catalysts exhibit superior durability and longer lifespan compared to aluminum-based catalysts due to their excellent resistance to oxidation and high-temperature stability. Aluminum catalysts tend to degrade faster under harsh reaction conditions, leading to a reduction in catalytic activity and overall lifespan. The robust chemical inertness of platinum ensures sustained catalytic performance in industrial applications, making it a preferred choice despite higher initial costs.
Availability and Sourcing
Aluminum is abundant in the Earth's crust, making it highly available and cost-effective for catalytic applications, whereas platinum is a rare and precious metal, significantly limited in supply and expensive to source. The widespread availability of aluminum enables large-scale industrial use, while platinum's scarcity restricts its use to high-value or specialized catalytic processes. Mining and refining platinum involves complex, environmentally intensive methods, contrasting with the more straightforward sourcing of aluminum from bauxite ore.
Recent Advances in Catalyst Technology
Recent advances in catalyst technology highlight the superior thermal stability and electron transfer efficiency of platinum catalysts compared to aluminum-based catalysts, significantly enhancing reaction rates in fuel cells and automotive emissions control. Nanostructured platinum materials with optimized morphology exhibit increased active surface area, improving catalytic performance while reducing precious metal usage. Innovations in aluminum oxide supports improve metal dispersion and resistance to sintering, offering cost-effective alternatives for industrial catalytic processes.
Conclusion: Choosing the Right Catalyst
Aluminum catalysts offer cost-effectiveness and high availability, making them suitable for large-scale industrial processes with moderate activity requirements. Platinum catalysts, despite their higher price, deliver superior catalytic efficiency and durability, ideal for precision applications demanding high performance and longevity. Selecting the right catalyst depends on balancing budget constraints with desired catalytic activity and process stability.
Infographic: Aluminum vs Platinum for Catalyst
 azmater.com
azmater.com