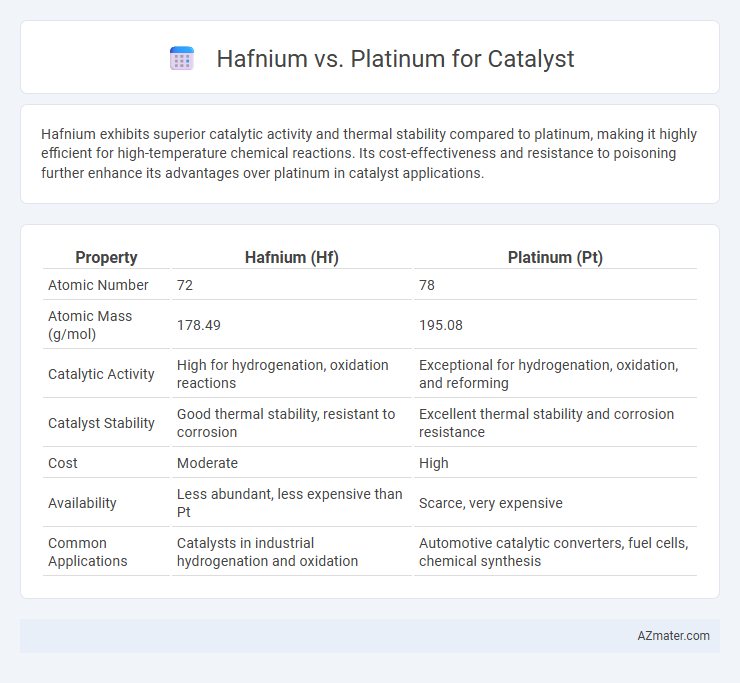
Hafnium exhibits superior catalytic activity and thermal stability compared to platinum, making it highly efficient for high-temperature chemical reactions. Its cost-effectiveness and resistance to poisoning further enhance its advantages over platinum in catalyst applications.
Table of Comparison
| Property | Hafnium (Hf) | Platinum (Pt) |
|---|---|---|
| Atomic Number | 72 | 78 |
| Atomic Mass (g/mol) | 178.49 | 195.08 |
| Catalytic Activity | High for hydrogenation, oxidation reactions | Exceptional for hydrogenation, oxidation, and reforming |
| Catalyst Stability | Good thermal stability, resistant to corrosion | Excellent thermal stability and corrosion resistance |
| Cost | Moderate | High |
| Availability | Less abundant, less expensive than Pt | Scarce, very expensive |
| Common Applications | Catalysts in industrial hydrogenation and oxidation | Automotive catalytic converters, fuel cells, chemical synthesis |
Introduction to Hafnium and Platinum as Catalysts
Hafnium and platinum serve distinct roles as catalysts based on their unique electronic structures and chemical properties. Hafnium, a transition metal in group 4, exhibits high thermal stability and strong resistance to corrosion, making it effective in catalytic processes involving hydrogenation and hydrocarbon reforming. Platinum, a precious metal widely used in catalytic converters and fuel cells, offers exceptional catalytic activity and selectivity due to its ability to adsorb and activate a wide range of molecules under mild conditions.
Chemical Properties: Hafnium vs Platinum
Hafnium exhibits high thermal stability and strong resistance to corrosion, making it suitable for high-temperature catalytic processes, whereas platinum demonstrates exceptional catalytic activity and selectivity due to its unique electron configuration and surface chemistry. Platinum's ability to adsorb and activate hydrogen and oxygen molecules efficiently contrasts with hafnium's limited catalytic activity but superior durability under aggressive chemical environments. The difference in electronic structure, where platinum's d-orbitals facilitate surface reactions, highlights why platinum remains the preferred catalyst in many industrial applications despite hafnium's robustness.
Catalytic Efficiency Comparison
Hafnium catalysts exhibit high thermal stability and enhanced resistance to coking, making them suitable for high-temperature reactions, while platinum catalysts are renowned for their exceptional catalytic activity and selectivity in hydrogenation and oxidation processes. Platinum generally demonstrates superior catalytic efficiency due to its ability to facilitate rapid electron transfer and lower activation energies, resulting in higher reaction rates under mild conditions. However, hafnium's cost-effectiveness and durability offer advantages in specific industrial catalytic applications where catalyst longevity and operating environment are critical factors.
Cost and Availability Analysis
Hafnium offers a significantly lower cost compared to platinum, making it an economically attractive alternative for catalyst applications. While platinum is rare and highly valuable due to its limited natural reserves, hafnium is more abundant, mined primarily as a byproduct of zirconium production. The greater availability of hafnium reduces supply chain risks and price volatility commonly associated with platinum catalysts.
Stability and Longevity of Hafnium and Platinum Catalysts
Hafnium catalysts exhibit exceptional thermal stability and resistance to oxidation, maintaining performance over prolonged periods in harsh reaction environments. Platinum catalysts, while highly active and widely used, often face deactivation due to sintering and poisoning under high-temperature or contaminant exposure. The superior longevity of hafnium catalysts in corrosive or high-temperature processes makes them increasingly attractive for sustainable industrial applications.
Environmental Impact of Catalyst Usage
Hafnium catalysts exhibit lower environmental toxicity compared to platinum, reducing harmful metal leaching into ecosystems during industrial processes. Platinum catalysts, while highly efficient, often require energy-intensive extraction and recycling, leading to greater carbon emissions and resource depletion. The use of hafnium supports greener catalytic applications by minimizing hazardous waste and promoting sustainable chemical manufacturing.
Industrial Applications: Hafnium vs Platinum
Hafnium exhibits exceptional resistance to corrosion and high melting points, making it highly suitable for catalytic applications in extreme industrial environments such as nuclear reactors and high-temperature chemical processing. Platinum, renowned for its superior catalytic activity and stability, remains the preferred choice in automotive catalytic converters and pharmaceutical synthesis due to its ability to efficiently facilitate oxidation and hydrogenation reactions. While platinum's higher cost limits large-scale applications, hafnium's robustness offers an economically viable alternative in niche industries requiring durable catalysts under harsh conditions.
Recent Advances in Catalyst Technology
Recent advances in catalyst technology highlight the potential of hafnium as a cost-effective alternative to platinum in various catalytic applications, particularly in hydrogenation and electrocatalysis. Hafnium-based catalysts exhibit remarkable thermal stability and resistance to poisoning, enhancing their durability compared to conventional platinum catalysts. Emerging research emphasizes hafnium's ability to facilitate oxygen reduction and hydrogen evolution reactions with increased efficiency, positioning it as a promising material for sustainable energy solutions.
Challenges and Limitations of Each Metal
Hafnium catalysts face challenges such as limited availability and high cost, along with difficulties in achieving consistent catalytic activity due to surface oxidation and alloy formation. Platinum catalysts suffer from deactivation caused by poisoning with sulfur and carbon compounds, as well as sintering under high-temperature conditions leading to loss of active surface area. Both metals encounter scalability limitations, with platinum's rarity affecting commercial viability and hafnium's complex extraction impacting widespread industrial application.
Future Prospects in Catalyst Development
Hafnium's unique electronic properties and resistance to corrosion position it as a promising alternative to platinum in catalyst development, especially for applications requiring high thermal stability. Platinum remains the benchmark due to its exceptional catalytic efficiency in fuel cells and industrial processes, but its scarcity and high cost drive research into hafnium-based catalysts. Future prospects include utilizing hafnium alloys and nanostructures to achieve enhanced catalytic performance and durability, potentially reducing reliance on platinum in sustainable energy technologies.
Infographic: Hafnium vs Platinum for Catalyst
 azmater.com
azmater.com