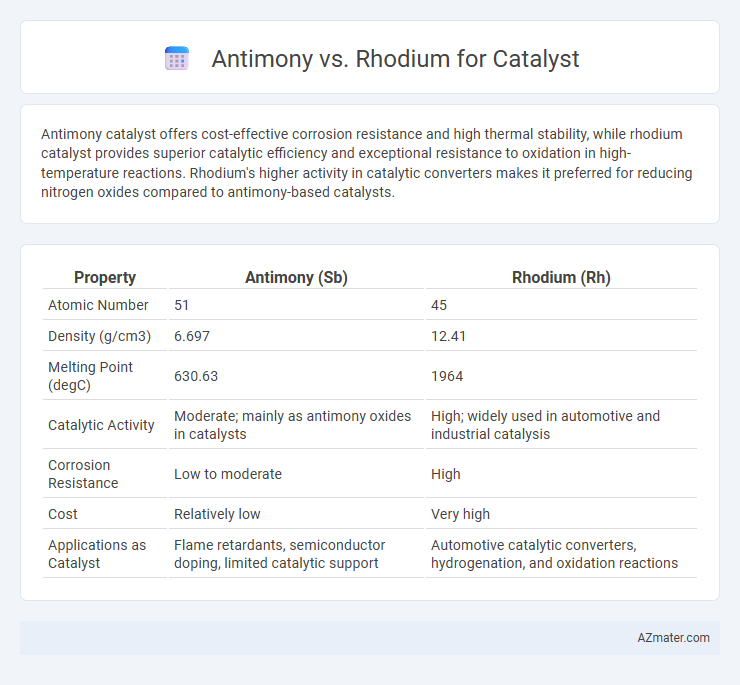
Antimony catalyst offers cost-effective corrosion resistance and high thermal stability, while rhodium catalyst provides superior catalytic efficiency and exceptional resistance to oxidation in high-temperature reactions. Rhodium's higher activity in catalytic converters makes it preferred for reducing nitrogen oxides compared to antimony-based catalysts.
Table of Comparison
| Property | Antimony (Sb) | Rhodium (Rh) |
|---|---|---|
| Atomic Number | 51 | 45 |
| Density (g/cm3) | 6.697 | 12.41 |
| Melting Point (degC) | 630.63 | 1964 |
| Catalytic Activity | Moderate; mainly as antimony oxides in catalysts | High; widely used in automotive and industrial catalysis |
| Corrosion Resistance | Low to moderate | High |
| Cost | Relatively low | Very high |
| Applications as Catalyst | Flame retardants, semiconductor doping, limited catalytic support | Automotive catalytic converters, hydrogenation, and oxidation reactions |
Introduction to Antimony and Rhodium as Catalysts
Antimony and rhodium serve as important catalysts in various chemical reactions, each exhibiting unique properties that influence their effectiveness. Antimony catalysts are valued for their ability to facilitate oxidation reactions and improve the stability of catalytic systems, particularly in the manufacture of polyethylene terephthalate (PET) and other polymers. Rhodium catalysts excel in hydrogenation and carbonylation processes due to their superior activity and selectivity, making them indispensable in automotive catalytic converters and fine chemical synthesis.
Chemical Properties Relevant to Catalysis
Antimony exhibits moderate catalytic activity due to its semimetallic nature, providing electron-rich sites that facilitate redox reactions, particularly in oxidation catalysts. Rhodium, a rare transition metal, offers exceptional catalytic properties such as high resistance to poisoning and excellent hydrogenation and carbon monoxide oxidation capabilities due to its d-band electronic structure. The distinct electron configurations and surface chemistries of antimony and rhodium significantly influence their catalytic efficiencies and selectivity in chemical processes.
Mechanisms of Catalytic Action
Antimony acts as a promoter in catalytic systems by modifying the electronic properties of active metals and enhancing resistance to poisoning, often facilitating oxidation-reduction reactions through electron transfer modulation. Rhodium serves as an active catalytic center with its d-electrons enabling adsorption and activation of reactant molecules, particularly in hydrogenation and carbon monoxide oxidation, by forming transient metal-adsorbate complexes. The mechanisms differ as antimony primarily influences catalyst stability and selectivity, whereas rhodium directly participates in bond breaking and forming via surface-mediated reaction pathways.
Efficiency and Selectivity Comparison
Rhodium catalysts demonstrate superior efficiency in hydrogenation reactions, achieving higher turnover frequencies and lower activation energies compared to antimony-based catalysts. Antimony exhibits enhanced selectivity in oxidation processes, favoring partial oxidation with minimized byproduct formation, whereas rhodium often promotes complete oxidation. The choice between antimony and rhodium catalysts hinges on the specific reaction pathway, balancing rhodium's efficiency with antimony's selective control in catalytic applications.
Cost Analysis and Economic Viability
Antimony catalysts generally offer a lower initial cost compared to rhodium, which is one of the most expensive precious metals due to its rarity and high demand in automotive catalytic converters. Rhodium's superior catalytic efficiency and durability can offset its higher price by reducing the frequency of catalyst replacement and improving overall process yields. Economic viability hinges on balancing upfront expenditure against long-term operational savings, with rhodium favored in high-performance applications despite its premium cost.
Environmental Impact and Sustainability
Antimony-based catalysts raise environmental concerns due to the element's toxicity and potential bioaccumulation, posing risks to ecosystems and human health during both production and disposal stages. Rhodium catalysts, while expensive, offer higher efficiency and recyclability, reducing waste generation and energy consumption in catalytic processes. Sustainable catalyst selection increasingly favors rhodium for its lower environmental footprint despite higher initial costs, aligning with green chemistry principles and stricter regulatory standards.
Industrial Applications and Use Cases
Antimony is primarily used as a catalyst promoter in industrial processes like polyethylene production and rubber vulcanization, enhancing catalyst efficiency and product quality. Rhodium serves as a superior catalyst in automotive catalytic converters and chemical manufacturing, particularly for hydrogenation and carbonylation reactions due to its excellent resistance to high temperatures and corrosion. Industrial applications favor rhodium for processes requiring high catalytic activity and durability, while antimony is chosen for cost-effective improvements in polymerization and oxidation reactions.
Catalyst Longevity and Stability
Rhodium catalysts are renowned for their exceptional longevity and stability under high-temperature and chemically aggressive conditions, making them ideal for industrial catalytic converters and hydrogenation reactions. Antimony catalysts, while useful in specific niche applications, generally exhibit lower thermal stability and tend to degrade faster when exposed to harsh reaction environments. The superior resistance of rhodium to sintering and poisoning ensures a prolonged catalytic lifespan compared to antimony-based catalysts.
Safety and Handling Considerations
Antimony catalysts pose significant safety challenges due to their toxicity and potential environmental hazards, requiring stringent handling protocols such as protective equipment and proper ventilation. Rhodium catalysts, while also expensive and requiring careful handling, are generally considered less toxic and have better chemical stability, reducing risks during catalyst preparation and use. Proper training and adherence to safety guidelines are critical to minimize exposure and ensure safe disposal when working with either antimony or rhodium in catalytic applications.
Future Trends in Catalyst Development
Antimony and rhodium serve distinct roles in catalyst development, with rhodium widely favored for its superior activity and selectivity in automotive catalytic converters. Future trends emphasize the search for cost-effective alternatives to rhodium due to its high price and scarcity, driving increased research into antimony-based catalysts and their potential for enhancing catalytic efficiency in green chemistry applications. Advances in nanostructuring and alloying techniques are expected to optimize antimony catalysts, promoting sustainability and reducing reliance on precious metals.
Infographic: Antimony vs Rhodium for Catalyst
 azmater.com
azmater.com