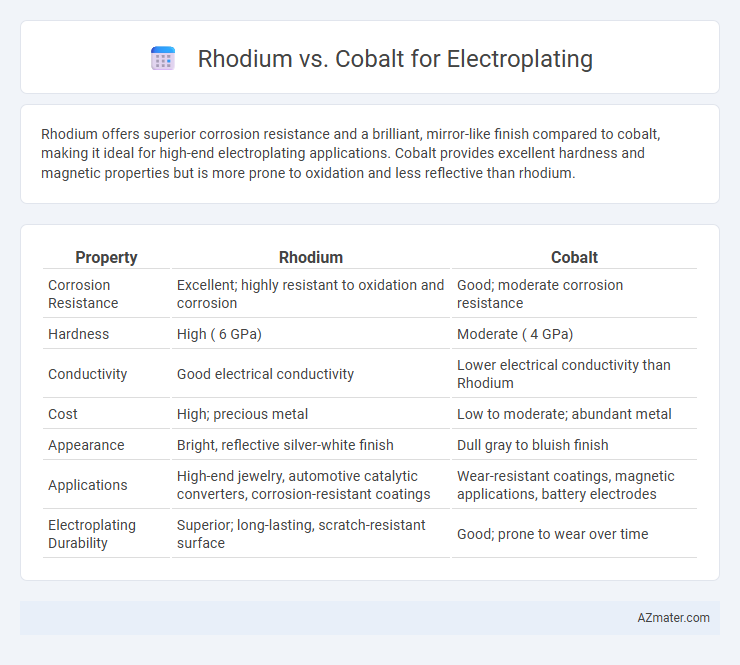
Rhodium offers superior corrosion resistance and a brilliant, mirror-like finish compared to cobalt, making it ideal for high-end electroplating applications. Cobalt provides excellent hardness and magnetic properties but is more prone to oxidation and less reflective than rhodium.
Table of Comparison
| Property | Rhodium | Cobalt |
|---|---|---|
| Corrosion Resistance | Excellent; highly resistant to oxidation and corrosion | Good; moderate corrosion resistance |
| Hardness | High ( 6 GPa) | Moderate ( 4 GPa) |
| Conductivity | Good electrical conductivity | Lower electrical conductivity than Rhodium |
| Cost | High; precious metal | Low to moderate; abundant metal |
| Appearance | Bright, reflective silver-white finish | Dull gray to bluish finish |
| Applications | High-end jewelry, automotive catalytic converters, corrosion-resistant coatings | Wear-resistant coatings, magnetic applications, battery electrodes |
| Electroplating Durability | Superior; long-lasting, scratch-resistant surface | Good; prone to wear over time |
Introduction to Rhodium and Cobalt in Electroplating
Rhodium and cobalt are critical metals used in electroplating for their distinct properties; rhodium offers exceptional corrosion resistance, high reflectivity, and an elegant bright finish, making it ideal for jewelry and automotive trim. Cobalt provides excellent wear resistance, hardness, and magnetic properties, widely applied in industrial tools and electronics coatings. The choice between rhodium and cobalt electroplating depends on factors like desired appearance, durability, and cost-effectiveness in specific applications.
Chemical Properties and Composition
Rhodium exhibits exceptional corrosion resistance and a high melting point of 1964degC, making it ideal for electroplating applications requiring durability and chemical stability, while its atomic number is 45 and it is a member of the platinum group metals. Cobalt, with an atomic number of 27 and a melting point of 1495degC, offers magnetic properties and good wear resistance but is more prone to oxidation than rhodium. The chemical composition difference, where rhodium is a noble metal with strong resistance to acids and cobalt is a transition metal more reactive in acidic environments, significantly influences their selection for specific electroplating purposes.
Electroplating Process: Rhodium vs Cobalt
The electroplating process for rhodium involves depositing a thin, highly reflective, and corrosion-resistant layer from a rhodium salt solution, typically rhodium chloride, using an acidic electrolyte. In contrast, cobalt electroplating uses cobalt salts in an acidic or neutral bath, producing a durable, wear-resistant coating with magnetic properties. Rhodium plating offers superior brightness and tarnish resistance, while cobalt plating is valued for hardness and adhesion in industrial applications.
Durability and Wear Resistance
Rhodium offers superior durability and wear resistance compared to cobalt, making it an ideal choice for electroplating applications requiring long-lasting, scratch-resistant surfaces. Its hardness and chemical inertness ensure that coated items maintain their luster and resist corrosion over extended use. Cobalt, while providing reasonable wear resistance, tends to be less durable under harsh conditions and may require more frequent re-plating to maintain performance.
Appearance and Surface Finish
Rhodium electroplating offers a bright, highly reflective, and mirror-like surface finish, providing superior corrosion resistance and a smooth, durable coating ideal for jewelry and decorative applications. Cobalt plating delivers a matte to semi-bright finish with excellent hardness and wear resistance, but it lacks the brilliant luster and high reflectivity characteristic of rhodium. The choice between rhodium and cobalt plating depends on the desired aesthetic effect, with rhodium favored for its bright, shiny appearance and cobalt selected for functional, abrasion-resistant surfaces.
Corrosion and Tarnish Resistance
Rhodium offers superior corrosion and tarnish resistance compared to cobalt due to its chemically inert nature and high resistance to oxidation in harsh environments. Cobalt coatings are more prone to corrosion and discoloration over time, especially when exposed to moisture and acidic conditions. For applications requiring long-lasting protection and a bright, tarnish-free finish, rhodium plating is the preferred choice.
Cost and Availability
Rhodium is significantly more expensive than cobalt due to its rarity and high demand in automotive and jewelry industries, with prices often exceeding $10,000 per ounce compared to cobalt's much lower market value around $25 per pound. Availability of rhodium is limited, primarily sourced from South Africa and Russia, causing supply chain volatility, while cobalt is more abundant, mainly mined in the Democratic Republic of Congo with stable global supply chains. Cost-effectiveness and accessibility make cobalt a preferred choice for large-scale electroplating, but rhodium remains unmatched for its superior corrosion resistance and reflectivity in high-end applications.
Applications in Various Industries
Rhodium electroplating is extensively used in automotive and jewelry industries for its exceptional corrosion resistance, high reflectivity, and wear protection. Cobalt electroplating finds applications in electronics, aerospace, and tooling industries due to its excellent hardness, magnetic properties, and thermal stability. While rhodium offers superior tarnish resistance and aesthetic appeal, cobalt is preferred for functional coatings requiring mechanical durability and heat resistance.
Environmental and Safety Considerations
Rhodium electroplating offers superior corrosion resistance and produces a highly reflective finish with lower toxicity compared to cobalt, which is associated with carcinogenic risks and environmental hazards during mining and processing. Cobalt electroplating requires careful handling due to its potential to release harmful cobalt dust and ions, necessitating strict safety protocols and protective equipment to minimize worker exposure. Selecting rhodium over cobalt reduces environmental impact and enhances workplace safety by limiting toxic metal pollution and hazardous waste generation.
Which is Better: Rhodium or Cobalt?
Rhodium offers superior corrosion resistance, exceptional brightness, and a hard, reflective finish ideal for high-end electroplating applications, especially in jewelry and electronics. Cobalt provides excellent wear resistance and toughness, making it more suitable for industrial coatings requiring durability under mechanical stress. Ultimately, rhodium is better for aesthetic and corrosion-resistant purposes, whereas cobalt excels in applications demanding hardness and longevity.
Infographic: Rhodium vs Cobalt for Electroplating
 azmater.com
azmater.com