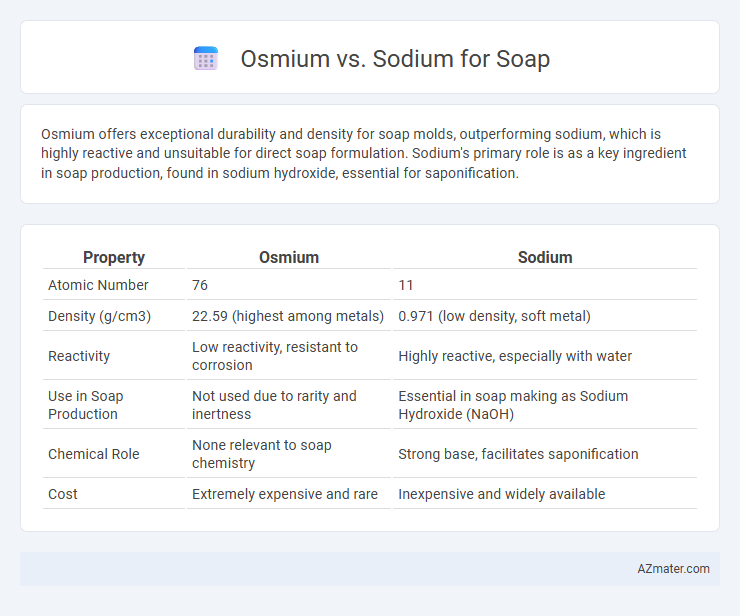
Osmium offers exceptional durability and density for soap molds, outperforming sodium, which is highly reactive and unsuitable for direct soap formulation. Sodium's primary role is as a key ingredient in soap production, found in sodium hydroxide, essential for saponification.
Table of Comparison
| Property | Osmium | Sodium |
|---|---|---|
| Atomic Number | 76 | 11 |
| Density (g/cm3) | 22.59 (highest among metals) | 0.971 (low density, soft metal) |
| Reactivity | Low reactivity, resistant to corrosion | Highly reactive, especially with water |
| Use in Soap Production | Not used due to rarity and inertness | Essential in soap making as Sodium Hydroxide (NaOH) |
| Chemical Role | None relevant to soap chemistry | Strong base, facilitates saponification |
| Cost | Extremely expensive and rare | Inexpensive and widely available |
Introduction to Osmium and Sodium in Soap Making
Osmium and sodium serve distinct roles in soap making, with sodium being the primary alkali used in traditional saponification to convert fats and oils into soap. Osmium, a dense and rare transition metal, is not commonly used in soap production due to its high cost and limited chemical reactivity in this context. The soap industry relies heavily on sodium hydroxide (NaOH) for its effectiveness in breaking down triglycerides, whereas osmium remains largely outside the scope of standard soap formulation.
Chemical Properties: Osmium vs Sodium
Osmium is a dense, hard transition metal with a high melting point and low reactivity, making it unsuitable for soap production, while sodium is an alkali metal known for its high reactivity and essential role in saponification. Sodium readily reacts with fatty acids to form soap through the hydrolysis of triglycerides, whereas osmium's inert nature prevents it from participating in such chemical reactions. The significant difference in their chemical properties highlights sodium's critical function in soap manufacturing compared to osmium's negligible role.
Reactivity in Soap Production Processes
Osmium and sodium differ significantly in reactivity during soap production, with sodium being highly reactive and essential for saponification by breaking down fats into soap and glycerin. Osmium exhibits minimal reactivity in this context, rendering it ineffective in soap manufacturing processes. Sodium's alkali properties enable efficient hydrolysis of triglycerides, positioning it as a key element in soap production over inert osmium.
Safety Considerations: Handling Osmium and Sodium
Handling osmium requires extreme caution due to its potential toxicity, particularly when inhaling osmium tetroxide fumes, which are highly volatile and hazardous. Sodium, a highly reactive alkali metal, poses significant fire and explosion risks upon contact with water or moisture, necessitating the use of inert atmospheres and protective gloves during handling. Proper ventilation, protective equipment, and adherence to strict safety protocols are essential when working with either element in soap production to prevent chemical burns, toxic exposure, or combustion incidents.
Lather Quality and Cleansing Power Comparison
Osmium is not used in soap formulation; sodium, typically in the form of sodium hydroxide or sodium salts, is essential for saponification, producing soap with effective cleansing power and rich lather. Sodium-based soaps generate a firm bar with abundant, stable bubbles that enhance the lather quality, effectively emulsifying oils and dirt for thorough cleansing. The chemical properties of sodium compounds ensure optimal removal of impurities and skin oils, whereas osmium's lack of surfactant properties makes it irrelevant to soap performance.
Cost and Availability for Soap Manufacturers
Osmium is an extremely rare and expensive metal, making it impractical and cost-prohibitive for soap manufacturers. Sodium, in contrast, is abundant and inexpensive, commonly used in the form of sodium hydroxide (lye) for saponification processes. The widespread availability and low cost of sodium compounds ensure scalability and economic efficiency in soap production.
Environmental Impact of Osmium and Sodium Soaps
Osmium soaps exhibit minimal environmental impact due to the element's extreme rarity and limited industrial use, resulting in negligible ecological disruption and low toxicity. In contrast, sodium-based soaps are widely produced and biodegradable but can contribute to water salinity and affect aquatic ecosystems when used in large quantities. The biodegradability and abundance of sodium soaps generally make them more environmentally sustainable compared to osmium-containing alternatives.
Skin Compatibility and Allergenic Potential
Osmium is not commonly used in soap formulations due to its rarity and high density, offering negligible benefits for skin compatibility, whereas sodium, primarily in the form of sodium hydroxide, is essential for saponification and generally safe when properly neutralized. Sodium-based soaps tend to have low allergenic potential, though individuals with sensitive skin may experience irritation from residual alkali or added fragrances. Osmium compounds are rarely evaluated for topical use and lack data on allergenic potential, making sodium the preferred element in soap for skin compatibility and safety.
Market Trends: Consumer Preference Analysis
Osmium-based soaps remain niche due to the metal's high cost and rarity, limiting widespread consumer adoption despite potential antimicrobial properties. Sodium, predominantly used as sodium hydroxide or sodium stearate in soap production, dominates the market due to affordability, effectiveness, and established supply chains. Current consumer preference strongly favors sodium-based soaps driven by cost-efficiency, biodegradability, and familiarity, while osmium soaps attract niche eco-conscious segments seeking innovative alternatives.
Conclusion: Choosing Between Osmium and Sodium for Soap
Sodium is the preferred choice for soap making due to its ability to produce hard, long-lasting bars with excellent cleansing properties. Osmium, being a rare and dense metal, has no practical application in soap formulation and offers no benefits in terms of saponification or skin compatibility. Selecting sodium-based compounds ensures effective, affordable, and safe soap products for everyday use.
Infographic: Osmium vs Sodium for Soap
 azmater.com
azmater.com