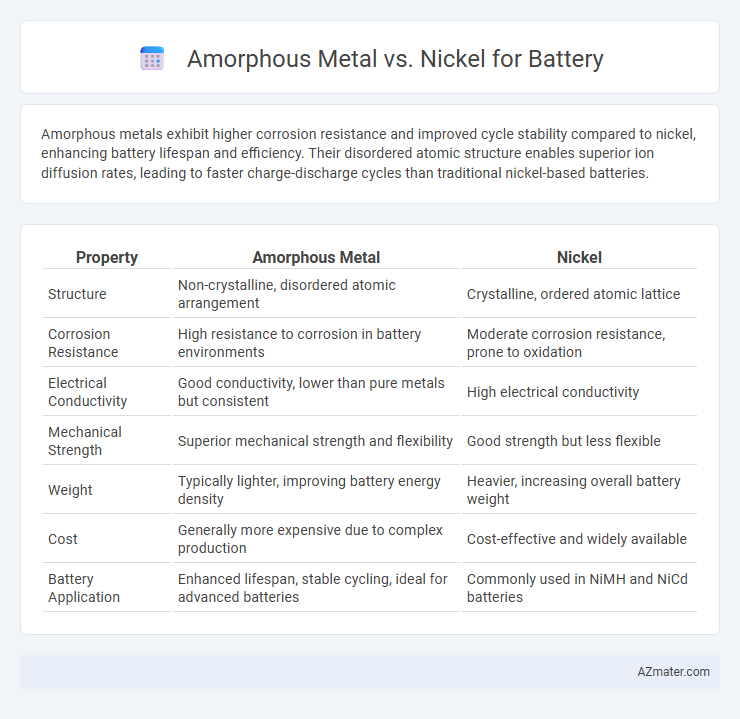
Amorphous metals exhibit higher corrosion resistance and improved cycle stability compared to nickel, enhancing battery lifespan and efficiency. Their disordered atomic structure enables superior ion diffusion rates, leading to faster charge-discharge cycles than traditional nickel-based batteries.
Table of Comparison
| Property | Amorphous Metal | Nickel |
|---|---|---|
| Structure | Non-crystalline, disordered atomic arrangement | Crystalline, ordered atomic lattice |
| Corrosion Resistance | High resistance to corrosion in battery environments | Moderate corrosion resistance, prone to oxidation |
| Electrical Conductivity | Good conductivity, lower than pure metals but consistent | High electrical conductivity |
| Mechanical Strength | Superior mechanical strength and flexibility | Good strength but less flexible |
| Weight | Typically lighter, improving battery energy density | Heavier, increasing overall battery weight |
| Cost | Generally more expensive due to complex production | Cost-effective and widely available |
| Battery Application | Enhanced lifespan, stable cycling, ideal for advanced batteries | Commonly used in NiMH and NiCd batteries |
Introduction: Comparing Amorphous Metal and Nickel in Batteries
Amorphous metals offer superior corrosion resistance and higher electrochemical stability compared to conventional nickel in battery electrodes, enhancing cycle life and energy density. Nickel remains widely used due to its high conductivity and cost-effectiveness, but amorphous metal alloys provide improved structural uniformity that reduces dendrite formation. The comparison highlights advancements in battery performance, where amorphous metals contribute to longer lifespan and safer operation in rechargeable cells.
Material Overview: Properties of Amorphous Metal
Amorphous metals, also known as metallic glasses, exhibit a disordered atomic structure that provides superior corrosion resistance and enhanced mechanical strength compared to crystalline metals like nickel. Their high electrical conductivity and unique magnetic properties make them advantageous for battery electrode applications, improving charge efficiency and cycle stability. The absence of grain boundaries in amorphous metals reduces internal friction and electrochemical degradation, leading to longer battery lifespan and better overall performance.
Nickel: Traditional Choice in Battery Technology
Nickel remains a traditional choice in battery technology due to its high energy density, excellent conductivity, and robust cycle life, making it ideal for nickel-cadmium (NiCd) and nickel-metal hydride (NiMH) batteries. Its stable electrochemical properties enable reliable performance in various applications, from portable electronics to electric vehicles. Although amorphous metals offer potential advantages in flexibility and corrosion resistance, nickel's well-established manufacturing processes and proven track record continue to dominate the battery market.
Electrochemical Performance: Efficiency and Capacity
Amorphous metals exhibit superior electrochemical performance in batteries compared to nickel, primarily due to their higher specific capacity and enhanced charge-discharge efficiency. The disordered atomic structure of amorphous metals facilitates faster ion diffusion and mitigates capacity fading during cycling, resulting in improved battery longevity and stability. Nickel, while offering good conductivity, generally shows lower capacity retention and efficiency under prolonged electrochemical stress.
Energy Density: Amorphous Metal vs Nickel
Amorphous metals exhibit higher energy density compared to nickel due to their disordered atomic structure, which allows for more efficient ion storage and faster charge-discharge cycles. Nickel, commonly used in battery electrodes, offers moderate energy density but often suffers from lower capacity retention and slower kinetics. The superior energy density of amorphous metals enhances battery performance, making them promising materials for high-capacity and long-lasting energy storage solutions.
Charge/Discharge Cycles and Longevity
Amorphous metals exhibit superior performance in battery charge/discharge cycles compared to nickel due to their disordered atomic structure, which reduces stress and degradation during cycling. This structural advantage leads to enhanced longevity and stability in battery applications, making amorphous metals a promising alternative to traditional nickel-based electrodes. Studies indicate that batteries using amorphous metal electrodes can achieve significantly higher cycle life and sustain capacity retention better than nickel counterparts under comparable operating conditions.
Thermal Stability and Safety Concerns
Amorphous metals exhibit superior thermal stability compared to nickel, maintaining structural integrity at higher temperatures and reducing the risk of thermal runaway in battery applications. The disordered atomic structure of amorphous metals allows for enhanced heat dissipation and lowers the chance of dendrite formation, which are common safety concerns associated with nickel-based batteries. Consequently, amorphous metal electrodes improve overall battery safety by minimizing overheating and enhancing cycle life under rigorous thermal conditions.
Cost Analysis and Economic Impact
Amorphous metals generally exhibit higher production costs than traditional nickel due to complex manufacturing processes and raw material scarcity, impacting initial battery investment significantly. Despite the cost premium, amorphous metals offer superior energy density and longer cycle life, potentially reducing total cost of ownership through extended battery lifespan and fewer replacements. Economic impact analysis shows that while nickel remains cost-effective for large-scale, short-term applications, amorphous metal batteries could drive long-term savings in sectors prioritizing performance and durability.
Environmental Sustainability and Recycling
Amorphous metal batteries offer enhanced environmental sustainability compared to nickel-based batteries due to their lower reliance on toxic and scarce raw materials, reducing ecological damage during extraction. The amorphous metal's structure facilitates easier recycling and material recovery, minimizing hazardous waste and energy-intensive processing. Nickel batteries, while effective, present challenges in recycling efficiency and often contribute to environmental pollution through mining and disposal processes.
Future Prospects and Emerging Applications
Amorphous metals exhibit superior corrosion resistance, higher strength, and excellent magnetic properties compared to nickel, making them promising for next-generation battery technologies like solid-state and lithium-sulfur cells. Their unique atomic structure enables enhanced charge-discharge cycles and thermal stability, positioning them as key materials in electric vehicle and renewable energy storage advancements. Emerging applications also explore amorphous metals in flexible and wearable batteries, leveraging their malleability and durability to meet growing demands in portable electronics.
Infographic: Amorphous metal vs Nickel for Battery
 azmater.com
azmater.com