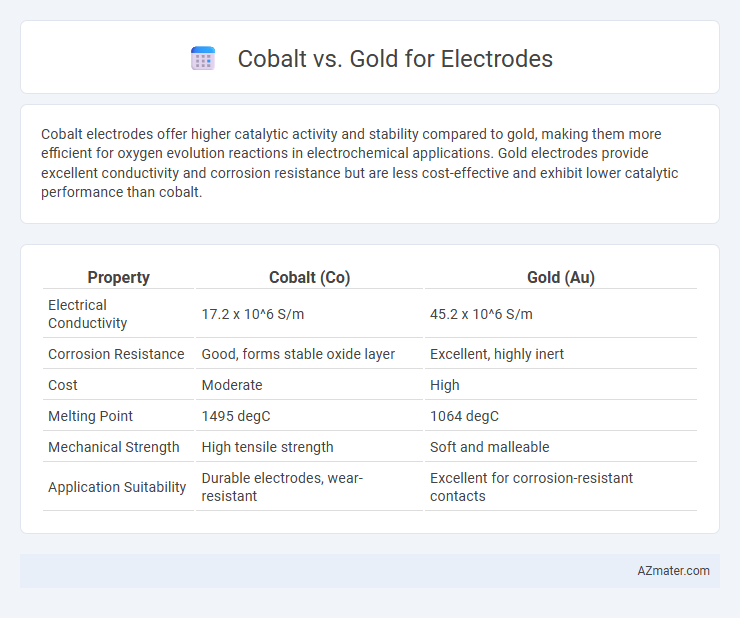
Cobalt electrodes offer higher catalytic activity and stability compared to gold, making them more efficient for oxygen evolution reactions in electrochemical applications. Gold electrodes provide excellent conductivity and corrosion resistance but are less cost-effective and exhibit lower catalytic performance than cobalt.
Table of Comparison
| Property | Cobalt (Co) | Gold (Au) |
|---|---|---|
| Electrical Conductivity | 17.2 x 10^6 S/m | 45.2 x 10^6 S/m |
| Corrosion Resistance | Good, forms stable oxide layer | Excellent, highly inert |
| Cost | Moderate | High |
| Melting Point | 1495 degC | 1064 degC |
| Mechanical Strength | High tensile strength | Soft and malleable |
| Application Suitability | Durable electrodes, wear-resistant | Excellent for corrosion-resistant contacts |
Introduction to Electrode Materials
Cobalt and gold are prominent materials used for electrodes due to their excellent electrical conductivity and chemical stability. Cobalt offers superior catalytic activity and cost-effectiveness, making it ideal for energy storage and electrocatalysis applications, while gold provides outstanding corrosion resistance and biocompatibility, essential in biomedical sensors and electronic devices. The choice between cobalt and gold electrodes depends on the specific performance requirements, environmental conditions, and economic considerations of the intended application.
Cobalt vs Gold: Overview and Applications
Cobalt electrodes demonstrate superior catalytic activity and corrosion resistance compared to gold, making them a preferred choice in energy storage systems like lithium-ion batteries and supercapacitors. Gold electrodes excel in conductivity and biocompatibility, often used in biosensors and medical devices where signal precision and stability are crucial. Applications of cobalt electrodes focus on high-performance energy solutions, whereas gold electrodes are favored in electronic and biomedical fields due to their inertness and excellent electrical properties.
Electrical Conductivity: Cobalt vs Gold
Gold exhibits superior electrical conductivity compared to cobalt, making it a preferred material for electrodes in high-performance electronic applications. Cobalt, while more affordable and mechanically robust, offers lower conductivity, which may result in increased resistance and energy loss. The conductivity of gold is approximately 45.2 x 10^6 S/m, significantly higher than cobalt's 17.2 x 10^6 S/m, highlighting gold's efficiency in electrical transmission.
Chemical Stability and Corrosion Resistance
Cobalt electrodes exhibit superior chemical stability in harsh electrochemical environments due to their robust oxide layer that resists degradation better than gold. While gold is highly inert and resistant to oxidation, cobalt provides enhanced corrosion resistance in alkaline and acidic media, making it suitable for more aggressive conditions. The choice between cobalt and gold electrodes hinges on the specific electrolyte composition and desired longevity under corrosive stress.
Cost Analysis: Economic Considerations
Cobalt electrodes generally offer a lower upfront cost compared to gold, making them more economically feasible for large-scale industrial applications. Gold, while significantly more expensive, provides superior conductivity and corrosion resistance, which may reduce long-term maintenance and replacement expenses. Cost analysis shows that the choice between cobalt and gold electrodes depends on balancing initial expenditure against operational durability and performance efficiency.
Fabrication and Ease of Processing
Cobalt electrodes exhibit superior adhesion and mechanical strength due to their robust fabrication methods such as sputtering and electroplating, enhancing durability in electrochemical applications. Gold electrodes provide excellent conductivity and chemical stability with simpler processing techniques like thermal evaporation, making them easier to fabricate for sensitive sensors. The choice between cobalt and gold depends on the balance between fabrication complexity and desired electrode performance, with cobalt favoring strength and gold favoring ease of processing.
Biocompatibility in Medical Devices
Cobalt electrodes demonstrate superior biocompatibility for medical devices due to their excellent corrosion resistance and lower ion release compared to gold, which minimizes potential cytotoxic effects. Gold electrodes, while highly conductive and chemically inert, can cause allergic reactions in some patients due to metal impurities and limited surface stability in physiological environments. Cobalt's ability to form stable oxide layers enhances tissue integration and reduces inflammatory responses, making it a preferred choice for implantable medical electrodes.
Performance in Energy Storage Solutions
Cobalt electrodes exhibit superior electrochemical stability and higher capacity retention compared to gold electrodes in energy storage solutions, making them ideal for high-performance batteries. Gold electrodes offer excellent conductivity and corrosion resistance but generally suffer from lower charge storage capacity and higher cost, limiting their practical application. The enhanced catalytic activity of cobalt significantly improves the energy density and cycle life of lithium-ion and supercapacitor systems.
Environmental Impact and Sustainability
Cobalt electrodes, commonly used in rechargeable batteries, pose significant environmental concerns due to toxic mining practices and human rights issues, leading to calls for more sustainable sourcing and recycling initiatives. Gold electrodes, although less common, offer superior conductivity and corrosion resistance but involve environmentally damaging extraction processes, including cyanide leaching and habitat destruction. Sustainable alternatives prioritize reducing cobalt reliance through material innovation and enhancing gold recovery from electronic waste to minimize ecological footprints.
Final Comparison: Choosing the Right Electrode Material
Cobalt electrodes offer superior conductivity and enhanced thermal stability compared to gold, making them ideal for high-performance electronic applications requiring durability under stress. Gold electrodes provide excellent corrosion resistance and biocompatibility, essential for medical devices and sensitive chemical sensors. Selecting the right electrode material depends on application-specific requirements such as electrical performance, environmental exposure, and long-term reliability.
Infographic: Cobalt vs Gold for Electrode
 azmater.com
azmater.com