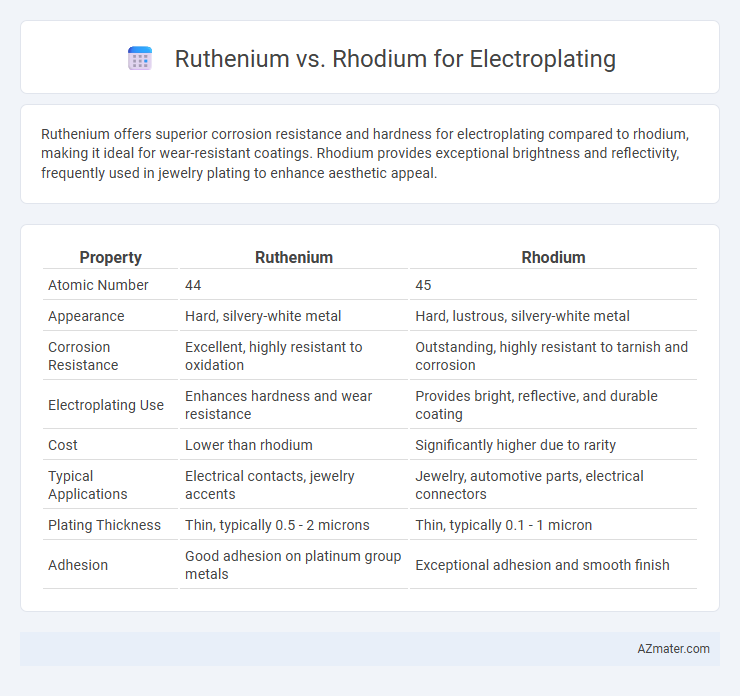
Ruthenium offers superior corrosion resistance and hardness for electroplating compared to rhodium, making it ideal for wear-resistant coatings. Rhodium provides exceptional brightness and reflectivity, frequently used in jewelry plating to enhance aesthetic appeal.
Table of Comparison
| Property | Ruthenium | Rhodium |
|---|---|---|
| Atomic Number | 44 | 45 |
| Appearance | Hard, silvery-white metal | Hard, lustrous, silvery-white metal |
| Corrosion Resistance | Excellent, highly resistant to oxidation | Outstanding, highly resistant to tarnish and corrosion |
| Electroplating Use | Enhances hardness and wear resistance | Provides bright, reflective, and durable coating |
| Cost | Lower than rhodium | Significantly higher due to rarity |
| Typical Applications | Electrical contacts, jewelry accents | Jewelry, automotive parts, electrical connectors |
| Plating Thickness | Thin, typically 0.5 - 2 microns | Thin, typically 0.1 - 1 micron |
| Adhesion | Good adhesion on platinum group metals | Exceptional adhesion and smooth finish |
Introduction to Ruthenium and Rhodium in Electroplating
Ruthenium and rhodium are precious metals belonging to the platinum group, widely used in electroplating for their exceptional corrosion resistance and hardness. Ruthenium electroplating provides a durable, wear-resistant coating often applied to electrical contacts and microelectronics, enhancing conductivity and extending component lifespan. Rhodium electroplating is prized for its brilliant, reflective finish and superior anti-tarnish properties, making it ideal for jewelry, automotive parts, and optical instruments where aesthetic and protective qualities are critical.
Chemical Properties and Structure Comparison
Ruthenium and rhodium both belong to the platinum group metals and exhibit excellent corrosion resistance and catalytic properties, making them ideal for electroplating applications. Ruthenium has a hexagonal close-packed crystal structure, providing high hardness and wear resistance, while rhodium features a face-centered cubic structure, contributing to its superior reflectivity and ductility. Chemically, ruthenium is more prone to oxidation in harsh environments, whereas rhodium maintains exceptional chemical stability, resulting in more durable and tarnish-resistant plated surfaces.
Electroplating Process Differences
Ruthenium electroplating typically involves a chloride-based bath, offering excellent hardness and corrosion resistance with moderate deposition rates. Rhodium electroplating uses a sulfate or chloride bath and is prized for its bright, reflective finish and superior wear resistance, though it often requires higher current densities and shorter plating cycles. The key process differences lie in bath composition, operating voltage, and current efficiency, impacting the resulting coating's thickness, uniformity, and durability.
Hardness and Durability Analysis
Rhodium offers superior hardness with a Mohs rating of approximately 6 to 6.5, making it highly resistant to scratches and wear, while ruthenium exhibits a slightly lower hardness around 4 to 6.5 but excels in chemical stability and corrosion resistance. Ruthenium's durability is enhanced by its strong oxidation resistance, making it ideal for harsh environments, whereas rhodium's exceptional hardness contributes to a longer-lasting, protective, and tarnish-resistant electroplated surface. In electroplating applications emphasizing hardness and durability, rhodium provides a more robust protective layer, while ruthenium is preferred for its enhanced corrosion resistance under extreme conditions.
Corrosion and Tarnish Resistance
Ruthenium and rhodium both offer excellent corrosion and tarnish resistance for electroplating, with rhodium exhibiting superior tarnish resistance particularly in harsh environments. Ruthenium provides strong chemical stability and good wear resistance, making it suitable for applications requiring durability against corrosion. Rhodium's bright, reflective finish and higher resistance to oxidation make it the preferred choice for decorative plating that demands long-lasting luster and protection.
Aesthetic Qualities and Appearance
Ruthenium electroplating offers a deep, dark gray to black finish that enhances contrast and provides a bold, modern aesthetic ideal for luxury watches and jewelry. Rhodium plating delivers a bright, reflective white-silver appearance with exceptional brightness and high corrosion resistance, making it popular for fine jewelry and automotive trim. While rhodium's mirror-like shine emphasizes brilliance and cleanliness, ruthenium's unique metallic hue stands out for its distinctive, sophisticated look.
Cost and Market Availability
Ruthenium and rhodium are both precious metals used in electroplating, but rhodium typically commands a higher price due to its greater demand and rarity, impacting overall project costs. Ruthenium offers a more cost-effective alternative with comparable corrosion resistance and hardness, making it suitable for specialized applications where budget constraints exist. Market availability fluctuates for both metals, but rhodium's limited supply and industrial demand often result in tighter availability and price volatility compared to the relatively more accessible ruthenium.
Industrial and Jewelry Applications
Ruthenium and rhodium are both prized in electroplating for their corrosion resistance and brilliant finishes, with rhodium frequently preferred in jewelry for its superior brightness and hypoallergenic properties. In industrial applications, ruthenium is valued for its hardness and durability, enhancing wear resistance in electronic components and contacts. Rhodium's higher cost limits its use primarily to decorative finishes, while ruthenium offers a cost-effective alternative for protective coatings in high-performance industrial settings.
Environmental Impact and Safety
Ruthenium and rhodium are both used in electroplating for their corrosion resistance and aesthetic appeal, but ruthenium generally poses a lower environmental risk due to its relatively stable chemical properties and less toxic byproducts. Rhodium electroplating often involves the use of harsh chemicals and produces more hazardous waste, requiring stringent safety measures to protect workers and the environment. Choosing ruthenium can reduce chemical hazards and lower overall environmental impact during plating and waste disposal processes.
Choosing the Right Metal for Electroplating Needs
Rhodium offers superior corrosion resistance and a brilliant, mirror-like finish ideal for high-end jewelry and automotive trim electroplating. Ruthenium provides excellent hardness and wear resistance, making it suitable for electronic connectors and hard protective coatings. Selecting the right metal depends on application requirements, balancing cost, durability, and desired aesthetics for optimal electroplating performance.
Infographic: Ruthenium vs Rhodium for Electroplating
 azmater.com
azmater.com