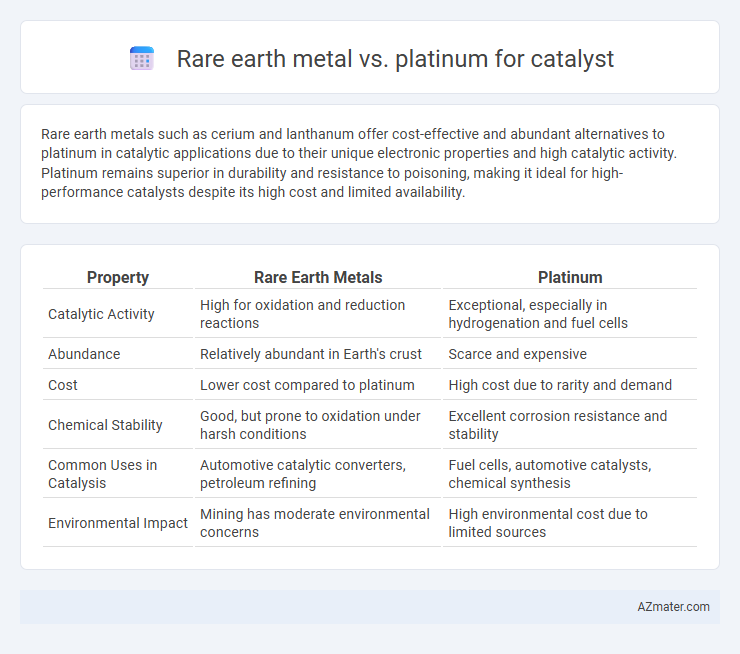
Rare earth metals such as cerium and lanthanum offer cost-effective and abundant alternatives to platinum in catalytic applications due to their unique electronic properties and high catalytic activity. Platinum remains superior in durability and resistance to poisoning, making it ideal for high-performance catalysts despite its high cost and limited availability.
Table of Comparison
| Property | Rare Earth Metals | Platinum |
|---|---|---|
| Catalytic Activity | High for oxidation and reduction reactions | Exceptional, especially in hydrogenation and fuel cells |
| Abundance | Relatively abundant in Earth's crust | Scarce and expensive |
| Cost | Lower cost compared to platinum | High cost due to rarity and demand |
| Chemical Stability | Good, but prone to oxidation under harsh conditions | Excellent corrosion resistance and stability |
| Common Uses in Catalysis | Automotive catalytic converters, petroleum refining | Fuel cells, automotive catalysts, chemical synthesis |
| Environmental Impact | Mining has moderate environmental concerns | High environmental cost due to limited sources |
Introduction to Catalysts: Rare Earth Metals vs Platinum
Rare earth metals and platinum both serve as critical catalysts in various industrial processes, with platinum widely recognized for its exceptional catalytic efficiency in automotive catalytic converters and fuel cells. Rare earth metals, such as cerium and lanthanum, offer unique catalytic properties, especially in oxidation-reduction reactions and as promoters in multi-component catalysts. The choice between rare earth metals and platinum depends on factors like cost, availability, catalytic activity, and stability under operating conditions.
Chemical Properties: Rare Earth Metals and Platinum
Rare earth metals exhibit unique chemical properties such as high reactivity and variable oxidation states, which enable efficient catalytic activity, especially in redox reactions. Platinum, a noble metal, is chemically stable with strong resistance to corrosion and oxidation, making it an ideal catalyst for hydrogenation and fuel cell applications. The combination of rare earth metals' ability to modify electronic environments and platinum's inherent catalytic durability enhances overall catalyst performance in various industrial processes.
Abundance and Availability
Rare earth metals such as cerium and lanthanum are more abundant in the Earth's crust, with estimated reserves exceeding 120 million metric tons, making them relatively accessible for large-scale catalyst production. In contrast, platinum, primarily sourced from South Africa and Russia, is significantly rarer, with global reserves estimated at around 69,000 metric tons, leading to higher costs and supply volatility. The greater abundance of rare earth metals supports their increasing use in catalytic converters and industrial processes, while platinum's scarcity drives ongoing research into alternative materials.
Catalytic Efficiency Comparison
Rare earth metals such as cerium and lanthanum exhibit exceptional catalytic efficiency in oxidation-reduction reactions due to their unique electronic structures and ability to cycle between multiple oxidation states. Platinum catalysts excel in hydrogenation and dehydrogenation processes, offering superior activity and selectivity, but can be limited by high cost and lower tolerance to sulfur poisoning. Comparing catalytic efficiency, rare earth metal catalysts often provide enhanced durability and cost-effectiveness in automotive exhaust systems, while platinum catalysts dominate in fine chemical synthesis and fuel cell technologies due to their unmatched surface reactivity.
Cost Analysis and Economic Considerations
Rare earth metals such as cerium and lanthanum significantly reduce catalyst costs compared to platinum, which is priced around $30,000 per kilogram, while rare earth prices typically range from $50 to $200 per kilogram. The economic viability of rare earth catalysts is enhanced by their abundance and lower extraction costs, leading to substantial savings in large-scale industrial applications. However, the long-term durability and performance efficiency of platinum catalysts often justify their higher initial investment in sectors where catalyst lifespan directly impacts overall operating expenses.
Environmental Impact and Sustainability
Rare earth metals and platinum are critical catalysts with distinct environmental footprints; rare earth mining often results in significant ecological disruption and toxic waste, whereas platinum extraction, though less abundant, involves energy-intensive processes with high carbon emissions. The recycling and recovery rates of platinum are higher due to established technologies, enhancing its sustainability compared to rare earth metals, which face challenges in efficient recycling and supply stability. Sustainable catalyst development prioritizes minimizing environmental impact by improving extraction methods and advancing recycling technologies for both rare earth metals and platinum.
Industrial Applications and Use Cases
Rare earth metals, such as cerium and lanthanum, are widely used in catalytic converters to reduce emissions in automotive and industrial exhaust systems due to their oxygen storage capacity and cost-effectiveness. Platinum, valued for its superior catalytic activity and durability, is essential in applications requiring high efficiency and resistance to poisoning, including fuel cells, petrochemical refining, and precise chemical synthesis processes. Industrial use cases highlight rare earth metals for large-scale, cost-sensitive pollution control, while platinum's premium performance suits critical, high-value catalytic reactions in energy and chemical industries.
Longevity and Durability in Catalytic Processes
Rare earth metals, such as cerium and lanthanum, exhibit exceptional oxygen storage capacity, enhancing catalyst longevity by maintaining active sites during redox cycles in catalytic converters. Platinum catalysts provide superior durability under high-temperature conditions but are prone to sintering and poisoning, which can reduce catalytic efficiency over time. The integration of rare earth metals alongside platinum improves overall catalyst durability, reducing degradation rates and extending functional lifespan in automotive and industrial catalytic processes.
Innovations and Recent Developments
Rare earth metals such as cerium and lanthanum have revolutionized catalytic converters by enhancing catalytic efficiency and reducing platinum group metal usage, leading to cost-effective and sustainable solutions. Recent developments in nano-structured rare earth catalysts demonstrate superior oxygen storage capacity and thermal stability compared to conventional platinum catalysts, driving advancements in automotive and chemical industries. Innovations in alloying rare earth elements with platinum group metals create hybrid catalysts with improved durability and catalytic performance under harsh reaction conditions.
Future Outlook: Rare Earth Metals vs Platinum Catalysts
The future outlook for catalysts highlights rare earth metals as promising alternatives to platinum due to their abundance and lower cost, driving innovation in sustainable catalytic processes. Rare earth metal catalysts exhibit unique electronic properties that enable enhanced activity and selectivity in chemical reactions, potentially surpassing platinum's performance in automotive and industrial applications. Continued advancements in material science and recycling technologies are expected to improve the viability and environmental footprint of rare earth metal catalysts, shaping a more cost-effective and resilient catalyst market.
Infographic: Rare earth metal vs Platinum for Catalyst
 azmater.com
azmater.com