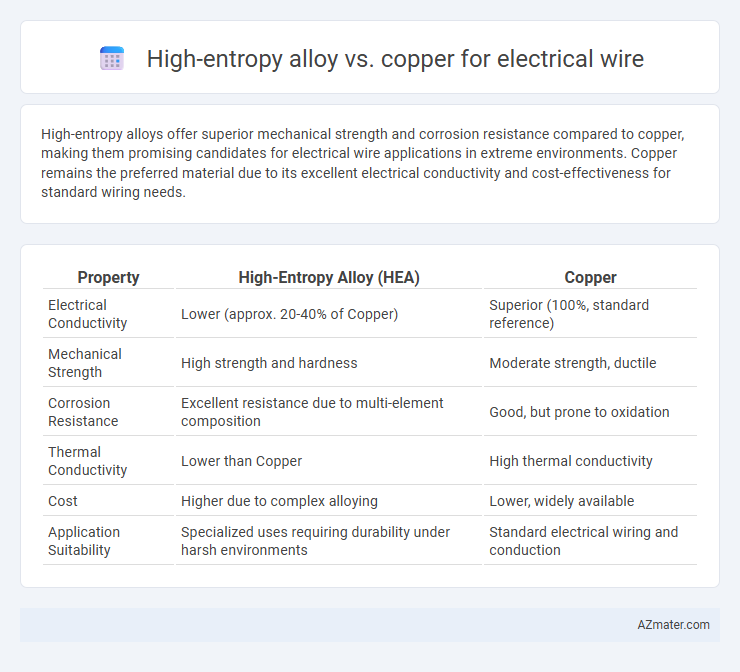
High-entropy alloys offer superior mechanical strength and corrosion resistance compared to copper, making them promising candidates for electrical wire applications in extreme environments. Copper remains the preferred material due to its excellent electrical conductivity and cost-effectiveness for standard wiring needs.
Table of Comparison
| Property | High-Entropy Alloy (HEA) | Copper |
|---|---|---|
| Electrical Conductivity | Lower (approx. 20-40% of Copper) | Superior (100%, standard reference) |
| Mechanical Strength | High strength and hardness | Moderate strength, ductile |
| Corrosion Resistance | Excellent resistance due to multi-element composition | Good, but prone to oxidation |
| Thermal Conductivity | Lower than Copper | High thermal conductivity |
| Cost | Higher due to complex alloying | Lower, widely available |
| Application Suitability | Specialized uses requiring durability under harsh environments | Standard electrical wiring and conduction |
Introduction to High-Entropy Alloys and Copper in Electrical Wiring
High-entropy alloys (HEAs) consist of multiple principal elements in near-equal proportions, offering exceptional mechanical strength, corrosion resistance, and thermal stability compared to traditional metals. Copper, a widely used electrical conductor, is valued for its excellent electrical conductivity, high ductility, and good thermal conductivity, making it the industry standard for electrical wiring. The emerging interest in HEAs for electrical wiring centers on their potential to combine high strength with adequate electrical conductivity, presenting an alternative to copper in demanding environments.
Composition and Structure: High-Entropy Alloys vs Copper
High-entropy alloys (HEAs) consist of multiple principal elements, typically five or more, in near-equal atomic percentages, creating a complex, multidimensional phase structure that enhances mechanical strength and corrosion resistance compared to pure copper's single-element face-centered cubic (FCC) lattice. The unique atomic-level disorder in HEAs disrupts electron scattering less than traditional alloys do, potentially improving electrical conductivity while maintaining superior thermal stability. In contrast, copper's simple, well-ordered FCC crystal structure offers excellent intrinsic conductivity but is limited by lower mechanical strength and thermal stability under extreme conditions.
Electrical Conductivity Comparison
High-entropy alloys (HEAs) generally exhibit lower electrical conductivity compared to pure copper due to increased electron scattering caused by their multi-element composition. Copper's electrical conductivity is approximately 5.96 x 10^7 S/m at room temperature, making it one of the best conductors for electrical wiring applications. While HEAs offer superior mechanical strength and corrosion resistance, copper remains the preferred material for electrical wires where high conductivity is critical.
Mechanical Strength and Durability
High-entropy alloys (HEAs) offer superior mechanical strength and enhanced durability compared to traditional copper when used in electrical wiring. Their multi-element composition provides exceptional resistance to wear, fatigue, and corrosion, ensuring longer service life under harsh conditions. Copper, while highly conductive, tends to exhibit lower tensile strength and can suffer from mechanical degradation over extended use, making HEAs a promising alternative for applications demanding robust performance.
Corrosion and Oxidation Resistance
High-entropy alloys exhibit superior corrosion and oxidation resistance compared to copper, making them ideal for harsh environments where long-term durability is critical. Copper tends to oxidize quickly, forming a layer of copper oxide that can reduce electrical conductivity, while high-entropy alloys maintain stable surface oxides that protect against further degradation. These properties ensure that high-entropy alloy wires offer enhanced performance and longevity in electrical applications exposed to moisture and corrosive agents.
Thermal Stability and Performance
High-entropy alloys (HEAs) exhibit superior thermal stability compared to traditional copper, maintaining structural integrity and electrical conductivity at elevated temperatures above 800degC, whereas copper typically begins to degrade around 400degC. The complex composition of HEAs reduces grain boundary mobility, enhancing performance under thermal stress in electrical wiring applications. Copper remains preferred for its high conductivity (~5.96 x 10^7 S/m), but HEAs offer promising alternatives for high-temperature environments requiring improved durability and reduced failure rates.
Cost and Economic Considerations
High-entropy alloys (HEAs) present higher initial material and manufacturing costs compared to traditional copper due to their complex composition and processing requirements. Copper remains economically favorable for electrical wires because of its abundant availability, established supply chain, and excellent electrical conductivity, which reduce overall lifecycle expenses. However, HEAs may offer cost benefits in specialized applications where enhanced mechanical properties and corrosion resistance reduce maintenance and replacement costs.
Manufacturing Processes and Scalability
High-entropy alloys (HEAs) involve complex manufacturing processes such as arc melting, powder metallurgy, and advanced additive manufacturing techniques, which often require precise control and longer production times compared to copper's well-established continuous casting and rolling methods. The scalability of HEA production is currently limited by material cost and process complexity, whereas copper benefits from mature large-scale manufacturing infrastructure and supply chains, enabling efficient mass production of electrical wires. As a result, copper remains the industry standard for electrical wire production due to its cost-effectiveness and manufacturing scalability, while HEAs are explored mainly for niche applications requiring enhanced mechanical or corrosion resistance.
Environmental Impact and Sustainability
High-entropy alloys (HEAs) offer significant environmental benefits over traditional copper in electrical wiring due to their enhanced durability and corrosion resistance, reducing the need for frequent replacements and lowering resource consumption. Copper extraction and processing generate substantial greenhouse gas emissions and cause habitat disruption, whereas HEAs can be engineered with more abundant and less environmentally damaging elements. Incorporating HEAs in electrical wires promotes sustainability by extending service life and minimizing ecological footprints associated with mining and refining copper.
Future Prospects and Industry Applications
High-entropy alloys (HEAs) offer promising future prospects for electrical wiring due to their superior mechanical strength, thermal stability, and corrosion resistance compared to traditional copper wires. Industry applications are expanding as HEAs enable the development of high-performance electrical components in aerospace, automotive, and power transmission sectors, where durability under extreme conditions is critical. The potential of HEAs to reduce weight and improve efficiency positions them as a transformative material in next-generation electrical wiring technologies.
Infographic: High-entropy alloy vs Copper for Electrical Wire
 azmater.com
azmater.com