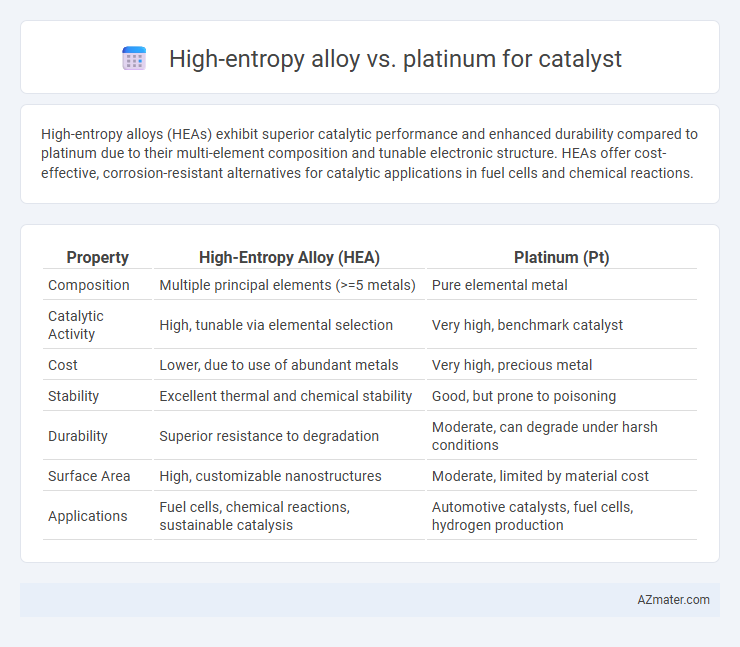
High-entropy alloys (HEAs) exhibit superior catalytic performance and enhanced durability compared to platinum due to their multi-element composition and tunable electronic structure. HEAs offer cost-effective, corrosion-resistant alternatives for catalytic applications in fuel cells and chemical reactions.
Table of Comparison
| Property | High-Entropy Alloy (HEA) | Platinum (Pt) |
|---|---|---|
| Composition | Multiple principal elements (>=5 metals) | Pure elemental metal |
| Catalytic Activity | High, tunable via elemental selection | Very high, benchmark catalyst |
| Cost | Lower, due to use of abundant metals | Very high, precious metal |
| Stability | Excellent thermal and chemical stability | Good, but prone to poisoning |
| Durability | Superior resistance to degradation | Moderate, can degrade under harsh conditions |
| Surface Area | High, customizable nanostructures | Moderate, limited by material cost |
| Applications | Fuel cells, chemical reactions, sustainable catalysis | Automotive catalysts, fuel cells, hydrogen production |
Introduction to Catalysts: High-Entropy Alloys vs Platinum
High-entropy alloys (HEAs) exhibit unique catalytic properties due to their multi-element composition and high configurational entropy, resulting in enhanced stability and activity across various reactions. Platinum, a noble metal, remains a benchmark catalyst known for exceptional catalytic efficiency, especially in fuel cells and hydrogen evolution reactions. Comparing HEAs to platinum highlights HEAs' potential to reduce precious metal usage while maintaining or surpassing catalytic performance under diverse environmental conditions.
Fundamental Properties of High-Entropy Alloys
High-entropy alloys (HEAs) exhibit superior catalytic performance compared to platinum due to their unique fundamental properties, including high configurational entropy, which stabilizes single-phase solid solutions and enhances thermal and chemical stability under reaction conditions. The multi-element composition of HEAs offers diverse active sites and tunable electronic structures, enabling improved adsorption and activation of reactants, leading to higher catalytic efficiency and selectivity. Unlike platinum, HEAs also demonstrate resistance to sintering and poisoning, making them promising alternatives for durable and cost-effective catalysts in energy conversion applications.
Platinum as a Benchmark Catalyst: Strengths and Limitations
Platinum remains the benchmark catalyst in various chemical reactions due to its exceptional catalytic activity and stability, particularly in processes like hydrogen evolution and oxygen reduction. Despite its strengths, platinum faces limitations such as high cost, scarcity, and susceptibility to poisoning, which restrict its widespread application. High-entropy alloys offer a promising alternative by providing tunable compositions that enhance catalytic performance and durability while potentially reducing reliance on precious metals like platinum.
Catalytic Efficiency: High-Entropy Alloys Compared to Platinum
High-entropy alloys (HEAs) demonstrate superior catalytic efficiency compared to platinum due to their unique multi-element compositions that create abundant active sites and synergistic effects. These alloys offer enhanced durability and resistance to poisoning, maintaining high performance in various catalytic reactions such as hydrogen evolution and oxygen reduction. Platinum's catalytic efficiency is often limited by its high cost and susceptibility to deactivation, whereas HEAs provide a cost-effective alternative with improved long-term stability and tunable surface properties.
Cost Analysis: Affordability of HEAs Versus Platinum
High-entropy alloys (HEAs) demonstrate significant cost advantages over platinum as catalysts due to the use of abundant, low-cost base metals reducing overall material expenses. The scalable synthesis of HEAs further enhances affordability by minimizing reliance on scarce and expensive platinum-group metals. Consequently, HEA catalysts provide a viable economic alternative for industrial applications requiring efficient and cost-effective catalytic materials.
Durability and Stability in Catalytic Applications
High-entropy alloys (HEAs) demonstrate superior durability and stability compared to platinum catalysts due to their multi-element composition, which enhances resistance to sintering, corrosion, and poisoning under harsh catalytic conditions. HEAs maintain structural integrity and catalytic activity over prolonged reaction cycles, outperforming platinum that often suffers from deactivation and particle agglomeration. Their unique atomic-level mixing enables continuous active site availability, making HEAs promising candidates for long-term catalytic applications in energy conversion and chemical synthesis.
Resistance to Poisoning and Deactivation
High-entropy alloys (HEAs) exhibit superior resistance to poisoning and deactivation compared to traditional platinum catalysts due to their multi-element compositions, which enhance surface stability and impede the adsorption of poisonous species like CO and sulfur compounds. Platinum catalysts, while highly active, are prone to rapid deactivation in the presence of contaminants, limiting their durability in harsh environments. The tunable nature of HEAs allows for tailored catalytic sites that maintain activity and longevity, making them more resilient for sustained applications in catalysis.
Environmental Impact and Sustainability Considerations
High-entropy alloys (HEAs) offer a promising alternative to platinum catalysts by utilizing abundant and less toxic elements, significantly reducing environmental impact and enhancing sustainability. The complex composition of HEAs enables superior catalytic performance with lower resource extraction demands compared to platinum, which is rare, expensive, and associated with environmentally damaging mining practices. Utilizing HEAs can lead to more sustainable catalytic processes in industries such as energy conversion and chemical manufacturing, aligning with global efforts to minimize ecological footprints and promote green technologies.
Applications: Where HEAs or Platinum Excel
High-entropy alloys (HEAs) excel in catalytic applications requiring high thermal stability and resistance to poisoning, such as in hydrogen evolution reactions and ammonia synthesis, due to their unique multi-element composition and tunable surface properties. Platinum remains a superior catalyst in fuel cells and automotive catalytic converters, offering unparalleled activity and selectivity for oxygen reduction and hydrocarbon oxidation reactions. While HEAs provide cost-effective, durable alternatives for harsh conditions, platinum ensures optimal performance in precision catalytic processes with well-established industrial usage.
Future Perspectives: Advancements and Challenges in Catalyst Development
High-entropy alloys (HEAs) offer promising future perspectives in catalyst development due to their tunable composition and exceptional stability under extreme conditions, providing a competitive alternative to traditional platinum catalysts. Advances in computational modeling and high-throughput experimentation enable the design of HEAs with optimized active sites for enhanced catalytic activity and selectivity, potentially reducing reliance on scarce and expensive platinum resources. Challenges remain in understanding the complex synergistic effects within multi-element HEAs and scaling up their synthesis for practical industrial applications, which are crucial for their widespread adoption in catalysis.
Infographic: High-entropy alloy vs Platinum for Catalyst
 azmater.com
azmater.com