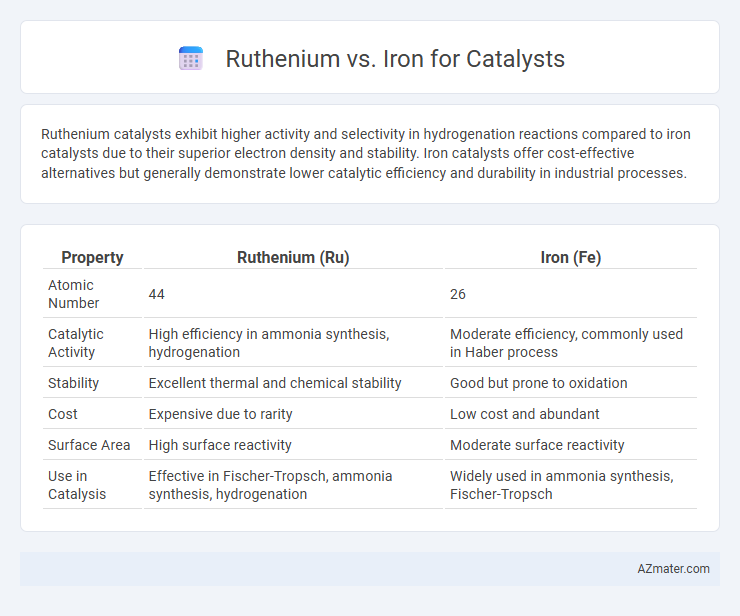
Ruthenium catalysts exhibit higher activity and selectivity in hydrogenation reactions compared to iron catalysts due to their superior electron density and stability. Iron catalysts offer cost-effective alternatives but generally demonstrate lower catalytic efficiency and durability in industrial processes.
Table of Comparison
| Property | Ruthenium (Ru) | Iron (Fe) |
|---|---|---|
| Atomic Number | 44 | 26 |
| Catalytic Activity | High efficiency in ammonia synthesis, hydrogenation | Moderate efficiency, commonly used in Haber process |
| Stability | Excellent thermal and chemical stability | Good but prone to oxidation |
| Cost | Expensive due to rarity | Low cost and abundant |
| Surface Area | High surface reactivity | Moderate surface reactivity |
| Use in Catalysis | Effective in Fischer-Tropsch, ammonia synthesis, hydrogenation | Widely used in ammonia synthesis, Fischer-Tropsch |
Introduction to Catalysts: Ruthenium and Iron
Ruthenium and iron are transition metals widely studied for their catalytic properties, with ruthenium known for high activity in hydrogenation and ammonia synthesis, while iron is abundant and cost-effective, commonly used in Fischer-Tropsch and Haber-Bosch processes. Ruthenium catalysts exhibit superior efficiency and selectivity due to their electronic configuration and ability to facilitate multi-electron transfers, whereas iron catalysts offer robustness and versatility in large-scale industrial applications. The choice between ruthenium and iron catalysts depends on specific reaction conditions, desired product purity, and economic considerations in catalyst design and deployment.
Chemical Properties: Ruthenium vs Iron
Ruthenium exhibits superior catalytic activity due to its higher electron density and ability to facilitate multiple oxidation states ranging from +2 to +8, which enhances its versatility in redox reactions compared to iron's common +2 and +3 states. Iron's lower electronegativity and higher prevalence in earth-abundant compounds make it cost-effective but less effective for specific catalytic oxidations and hydrogenations where ruthenium excels. The chemical stability of ruthenium under harsh reaction conditions, including resistance to oxidation and corrosion, contrasts with iron's susceptibility to rust and passivation, affecting catalyst longevity and performance.
Abundance and Cost Comparison
Ruthenium is significantly rarer than iron, with terrestrial abundance measured in parts per billion compared to iron's abundance at about 5% of the Earth's crust. This scarcity drives ruthenium prices to be substantially higher, often exceeding iron's cost by several orders of magnitude per kilogram. While ruthenium catalysts offer superior activity and selectivity in certain reactions, the abundant and cost-effective nature of iron makes it a preferred choice for large-scale, economical catalytic applications.
Catalytic Efficiency in Key Reactions
Ruthenium exhibits superior catalytic efficiency compared to iron in key reactions such as ammonia synthesis and hydrogenation due to its higher activity and stability under reaction conditions. Ruthenium catalysts achieve lower activation energies and exhibit greater resistance to sintering and poisoning, resulting in enhanced turnover frequencies and prolonged catalyst lifetimes. In contrast, iron catalysts, while cost-effective and abundant, often require higher temperatures and pressures, limiting their overall catalytic performance in industrial applications.
Selectivity and Reaction Specificity
Ruthenium catalysts exhibit higher selectivity and reaction specificity than iron in hydrogenation and ammonia synthesis due to their unique electronic properties and surface adsorption characteristics. Ruthenium's ability to facilitate specific bond activation leads to fewer side reactions and improved product purity, particularly in processes like Fischer-Tropsch synthesis. Iron catalysts, while cost-effective and abundant, typically show broader reaction pathways with lower selectivity, resulting in a mix of products and reduced overall catalyst efficiency.
Stability and Longevity of Catalysts
Ruthenium catalysts exhibit superior stability and longevity compared to iron catalysts due to their enhanced resistance to oxidation and thermal degradation. The robust electronic structure of ruthenium facilitates sustained catalytic activity under harsh reaction conditions, reducing catalyst deactivation rates. In contrast, iron catalysts often suffer from rapid oxidation and structural changes, limiting their operational lifespan and effectiveness in long-term applications.
Environmental Impact and Toxicity
Ruthenium catalysts exhibit lower environmental toxicity compared to iron due to their higher stability and resistance to corrosion, resulting in less metal leaching into ecosystems. Iron catalysts, while abundant and inexpensive, often pose greater ecological risks because their degradation products can contribute to soil and water contamination. The choice of ruthenium over iron in catalytic applications supports greener processes by minimizing hazardous waste and reducing overall environmental footprint.
Industrial Applications of Ruthenium and Iron Catalysts
Ruthenium catalysts are extensively used in ammonia synthesis, Fischer-Tropsch reactions, and hydrogenation processes due to their high activity and resistance to poisoning, making them ideal for industrial applications requiring efficient and durable catalysts. Iron catalysts dominate in large-scale Haber-Bosch processes for ammonia production because of their cost-effectiveness, abundance, and acceptable catalytic performance under high-pressure conditions. The selection between ruthenium and iron catalysts in industries often balances catalytic efficiency, operational conditions, and economic factors, with ruthenium preferred for specialized, high-performance applications and iron favored for bulk chemical manufacturing.
Recent Research and Innovations
Recent research highlights ruthenium's superior catalytic efficiency and selectivity compared to iron in hydrogenation and ammonia synthesis reactions, driven by its unique electronic properties and higher resistance to deactivation. Innovations in nanoparticle synthesis and support materials have significantly enhanced ruthenium's surface area and active site exposure, leading to improved catalytic performance in fuel cells and renewable energy applications. Iron catalysts remain attractive for cost-effective large-scale processes but often require complex promoters and harsh conditions to approach ruthenium's activity, prompting ongoing investigations into hybrid and alloy systems.
Future Trends in Catalyst Development
Ruthenium exhibits superior catalytic activity and stability in hydrogenation and ammonia synthesis compared to iron, making it a key focus for advanced catalyst development. Future trends emphasize ruthenium-based catalysts integrated with nanostructured supports to enhance selectivity and reduce precious metal usage. Iron catalysts remain relevant in large-scale processes due to cost-efficiency, but innovations in iron-based alloy catalysts aim to improve durability and catalytic efficiency in sustainable energy applications.
Infographic: Ruthenium vs Iron for Catalyst
 azmater.com
azmater.com