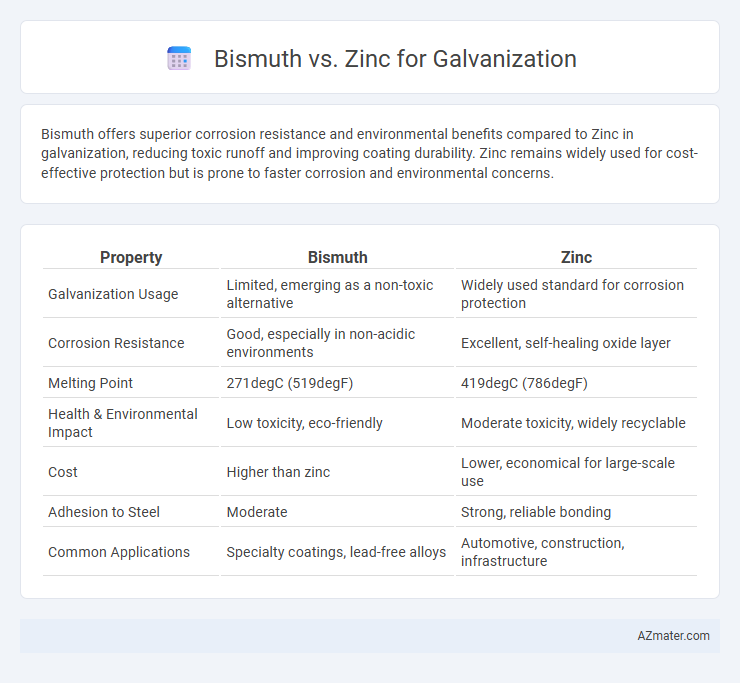
Bismuth offers superior corrosion resistance and environmental benefits compared to Zinc in galvanization, reducing toxic runoff and improving coating durability. Zinc remains widely used for cost-effective protection but is prone to faster corrosion and environmental concerns.
Table of Comparison
| Property | Bismuth | Zinc |
|---|---|---|
| Galvanization Usage | Limited, emerging as a non-toxic alternative | Widely used standard for corrosion protection |
| Corrosion Resistance | Good, especially in non-acidic environments | Excellent, self-healing oxide layer |
| Melting Point | 271degC (519degF) | 419degC (786degF) |
| Health & Environmental Impact | Low toxicity, eco-friendly | Moderate toxicity, widely recyclable |
| Cost | Higher than zinc | Lower, economical for large-scale use |
| Adhesion to Steel | Moderate | Strong, reliable bonding |
| Common Applications | Specialty coatings, lead-free alloys | Automotive, construction, infrastructure |
Introduction to Galvanization
Galvanization involves coating metals, primarily steel or iron, with a protective layer to prevent corrosion, typically using zinc due to its superior sacrificial properties and cost-effectiveness. Bismuth, while less common, offers environmental benefits and lower toxicity but lacks the extensive industrial application and proven durability of zinc in traditional galvanization processes. Zinc's widespread use in galvanization is driven by its ability to form a stable, adherent alloy layer that significantly extends the lifespan of metal structures exposed to harsh environments.
Why Consider Alternatives to Zinc in Galvanization?
Zinc has been the primary metal for galvanization due to its excellent corrosion resistance and affordability, but concerns over its environmental impact and limited supply are driving the search for alternatives like bismuth. Bismuth offers lower toxicity and a smaller ecological footprint, making it a promising substitute in coatings and corrosion protection. Exploring bismuth's properties can enhance galvanization sustainability while maintaining effective metal protection.
Overview of Zinc Galvanization
Zinc galvanization is a widely used method for protecting steel and iron from corrosion by applying a zinc coating that acts as a sacrificial anode, preventing rust formation. Compared to bismuth coatings, zinc offers superior durability, cost-effectiveness, and extensive industry acceptance due to its excellent corrosion resistance and well-established application processes. The galvanization process involving zinc typically includes hot-dip galvanizing or electro-galvanizing, ensuring enhanced longevity of metal structures in various environmental conditions.
Properties and Benefits of Bismuth in Galvanization
Bismuth offers superior corrosion resistance and low toxicity compared to zinc in galvanization, making it an environmentally friendly choice for protective coatings. Its low melting point and excellent wetting properties enable uniform metal deposition, improving coating adhesion and durability. Bismuth galvanization reduces health risks and environmental impact while maintaining effective protection against rust and wear.
Bismuth vs Zinc: Corrosion Resistance Comparison
Bismuth offers superior corrosion resistance compared to zinc in galvanization applications due to its stable oxide layer that protects underlying metals from moisture and chemical exposure. Zinc, while effective as a sacrificial anode in galvanization, can corrode more rapidly in highly acidic or saline environments, leading to reduced longevity of the protective coating. Choosing bismuth over zinc enhances durability in corrosive conditions, making it ideal for industrial components exposed to harsh chemicals or marine atmospheres.
Environmental Impact: Bismuth vs Zinc
Bismuth offers a lower environmental impact than zinc in galvanization due to its non-toxic and biodegradable properties, reducing soil and water contamination risks. Zinc mining and galvanizing processes contribute to heavy metal pollution and ecosystem disruption, causing harm to aquatic life and plants. Choosing bismuth as a coating metal enhances sustainability by minimizing hazardous waste and promoting eco-friendly industrial practices.
Cost Analysis of Bismuth and Zinc Galvanization
Bismuth galvanization generally incurs higher costs compared to zinc due to its relative scarcity and complex processing requirements, leading to increased raw material and production expenses. Zinc remains the more cost-effective option for galvanization, benefiting from abundant availability and established, efficient manufacturing processes that lower overall operational costs. When evaluating total expenditure, zinc's affordability and large-scale supply chain make it the preferred choice for economical corrosion protection in industrial applications.
Industrial Applications: Bismuth vs Zinc
Bismuth and zinc serve distinct roles in industrial galvanization, with zinc being the predominant choice due to its superior corrosion resistance and cost-effectiveness for protecting steel structures. Bismuth offers niche applications in specialized coatings owing to its non-toxic and environmentally friendly properties, making it suitable for use in industries requiring lead-free alternatives. Zinc's extensive industrial adoption spans automotive, construction, and infrastructure sectors, whereas bismuth's use is limited but growing in high-end, eco-conscious galvanization processes.
Challenges and Limitations of Bismuth Galvanization
Bismuth galvanization faces challenges such as higher material costs and limited availability compared to zinc, impacting its economic viability for large-scale industrial use. The lower melting point of bismuth results in reduced coating durability and weaker corrosion resistance under harsh environmental conditions. Additionally, compatibility issues with standard galvanizing equipment and processes limit the widespread adoption of bismuth coatings in galvanization applications.
Future Trends in Galvanization: Bismuth or Zinc?
Future trends in galvanization are increasingly exploring bismuth as a potential alternative to traditional zinc coatings due to its superior corrosion resistance and lower environmental impact. Advances in metallurgical processes enhance bismuth's adhesion and longevity, positioning it as a viable option for high-performance industrial applications. Zinc remains dominant owing to its cost-effectiveness and well-established supply chain, but ongoing research aims to optimize bismuth-zinc alloy coatings for improved durability and sustainability.
Infographic: Bismuth vs Zinc for Galvanization
 azmater.com
azmater.com