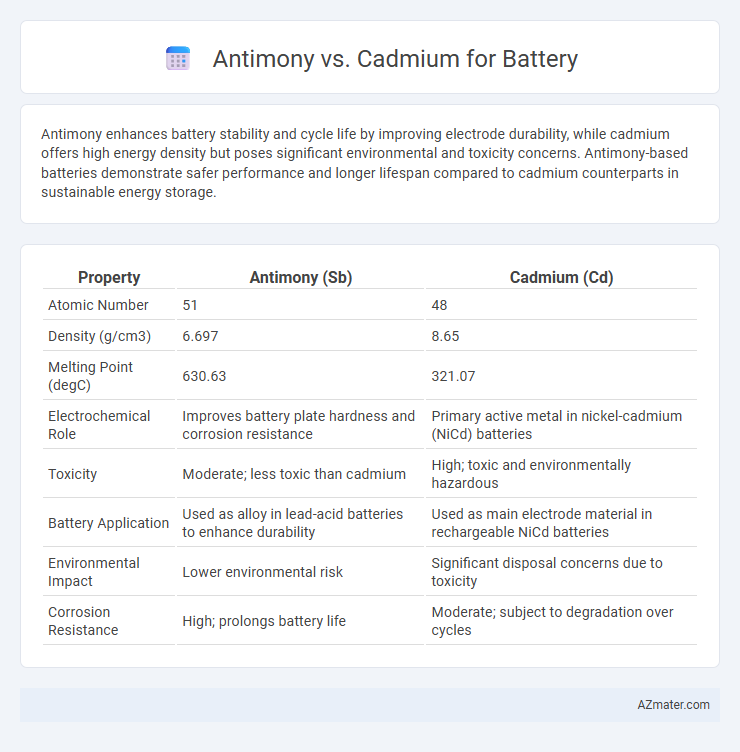
Antimony enhances battery stability and cycle life by improving electrode durability, while cadmium offers high energy density but poses significant environmental and toxicity concerns. Antimony-based batteries demonstrate safer performance and longer lifespan compared to cadmium counterparts in sustainable energy storage.
Table of Comparison
| Property | Antimony (Sb) | Cadmium (Cd) |
|---|---|---|
| Atomic Number | 51 | 48 |
| Density (g/cm3) | 6.697 | 8.65 |
| Melting Point (degC) | 630.63 | 321.07 |
| Electrochemical Role | Improves battery plate hardness and corrosion resistance | Primary active metal in nickel-cadmium (NiCd) batteries |
| Toxicity | Moderate; less toxic than cadmium | High; toxic and environmentally hazardous |
| Battery Application | Used as alloy in lead-acid batteries to enhance durability | Used as main electrode material in rechargeable NiCd batteries |
| Environmental Impact | Lower environmental risk | Significant disposal concerns due to toxicity |
| Corrosion Resistance | High; prolongs battery life | Moderate; subject to degradation over cycles |
Introduction to Antimony and Cadmium in Battery Technology
Antimony and cadmium are critical materials in battery technology, each offering unique electrochemical properties that influence energy storage performance. Antimony is primarily used in lead-acid and emerging sodium-ion batteries due to its ability to enhance electrode stability and increase cycle life. Cadmium, historically prevalent in nickel-cadmium (NiCd) batteries, provides excellent charge retention and durability, but faces reduced usage due to environmental and toxicity concerns.
Chemical Properties and Structure Comparison
Antimony and cadmium differ significantly in chemical properties and crystal structures that influence their battery applications. Antimony, a metalloid with atomic number 51, has a stable rhombohedral crystal structure and forms alloys with lithium, enhancing anode capacity and cycling stability in lithium-ion batteries. Cadmium, a transition metal with atomic number 48, has a hexagonal close-packed structure and exhibits higher electrical conductivity but lower capacity retention, making it primarily suited for nickel-cadmium batteries rather than lithium-based systems.
Historical Use of Antimony and Cadmium in Batteries
Antimony has been historically used as an alloying element in lead-acid battery grids to enhance mechanical strength and corrosion resistance, improving battery durability. Cadmium, primarily found in nickel-cadmium (NiCd) batteries, was widely utilized for its excellent electrochemical properties, enabling rechargeable performance and long cycle life. Both elements played critical roles during the 20th century in battery technology before environmental concerns limited cadmium usage and modern alternatives reduced antimony content.
Performance Metrics: Capacity, Efficiency, and Lifespan
Antimony exhibits higher theoretical capacity (660 mAh/g) compared to cadmium's lower capacity (~225 mAh/g), enhancing energy density in battery applications. Cadmium offers stable specific efficiency around 80-90%, but antimony-based batteries demonstrate improved cycle stability with capacity retention exceeding 70% after 500 cycles. Lifespan metrics favor antimony due to its alloying behavior with lithium, reducing volume expansion and enabling longer cycle life relative to cadmium, which suffers from faster capacity fading and shorter operational durability.
Environmental Impact and Toxicity
Antimony and cadmium both pose significant environmental and toxicity concerns when used in batteries; cadmium is highly toxic and classified as a carcinogen, causing severe harm to aquatic life and soil contamination during disposal or leakage. Antimony, while less toxic than cadmium, is still harmful, causing respiratory and skin irritation, and its compounds persist in the environment, bioaccumulating in organisms. The disposal and recycling of cadmium-based batteries require stringent regulations due to cadmium's severe toxicity, whereas antimony's environmental impact is comparatively moderate but still demands careful handling to prevent ecological damage.
Cost Analysis and Resource Availability
Antimony batteries offer a cost advantage due to the widespread availability and lower price of antimony compared to cadmium, whose extraction and refinement are more expensive and environmentally regulated. Cadmium-based batteries, while exhibiting high performance, face higher production costs driven by limited cadmium ore reserves and stringent environmental compliance requirements. Resource availability for antimony is relatively stable with significant deposits in China and Russia, whereas cadmium's scarcity and toxicological concerns restrict its supply, impacting overall battery cost and scalability.
Safety Considerations in Battery Applications
Antimony offers improved thermal stability and lower toxicity compared to cadmium, reducing safety risks in battery applications. Cadmium's high toxicity and environmental hazards necessitate stringent handling and disposal protocols to prevent health and ecological damage. Batteries utilizing antimony-based components exhibit enhanced safety profiles, making them preferable in applications requiring reliable and safe energy storage solutions.
Advancements in Antimony-Based and Cadmium-Based Batteries
Antimony-based batteries have seen significant advancements due to their higher theoretical capacity and improved cycle stability, making them promising candidates for next-generation energy storage. Cadmium-based batteries, while traditionally known for their robust performance and long-lasting cycles, face challenges related to toxicity and environmental concerns, which have spurred research into safer, more efficient alternatives. Innovations in nanostructuring and alloy composites have enhanced the electrochemical performance and durability of both antimony and cadmium electrodes, driving progress in high-capacity, cost-effective battery technologies.
Regulatory Policies Affecting Usage
Regulatory policies for antimony and cadmium in batteries vary significantly due to their environmental and health impacts. Cadmium faces stringent restrictions under the EU's Restriction of Hazardous Substances (RoHS) Directive and the Battery Directive, limiting its use to prevent toxic waste and human exposure. Antimony, while also regulated for toxicity under REACH (Registration, Evaluation, Authorisation and Restriction of Chemicals), generally encounters fewer restrictions, making it a more favorable element in battery manufacturing within current regulatory frameworks.
Future Prospects: Which Material Leads Battery Innovation?
Antimony offers promising advancements in battery technology due to its high theoretical capacity and excellent cycling stability, making it a strong candidate for next-generation sodium-ion and lithium-ion batteries. Cadmium's use in batteries is limited by toxicity and environmental concerns, despite its good electrical conductivity, which hinders its future application in sustainable energy storage. Current research trends favor antimony-based anodes, driven by scalable production techniques and better safety profiles, positioning antimony as the leading material in future battery innovation.
Infographic: Antimony vs Cadmium for Battery
 azmater.com
azmater.com