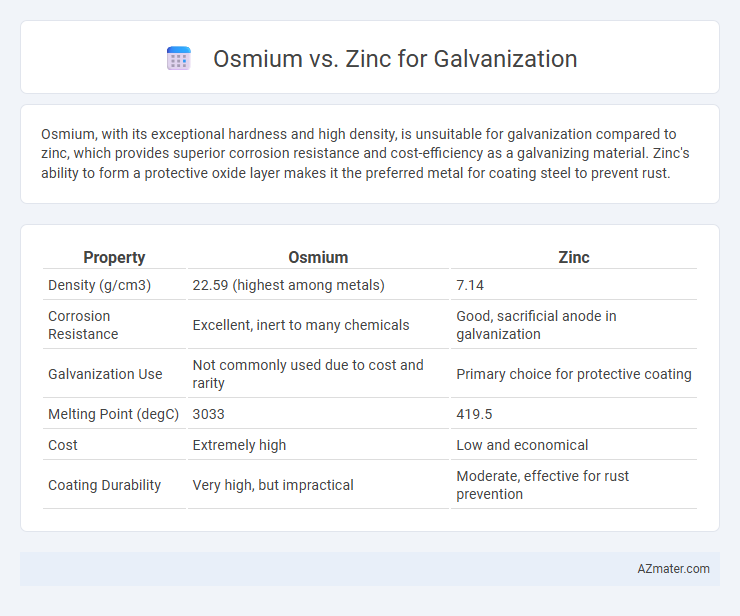
Osmium, with its exceptional hardness and high density, is unsuitable for galvanization compared to zinc, which provides superior corrosion resistance and cost-efficiency as a galvanizing material. Zinc's ability to form a protective oxide layer makes it the preferred metal for coating steel to prevent rust.
Table of Comparison
| Property | Osmium | Zinc |
|---|---|---|
| Density (g/cm3) | 22.59 (highest among metals) | 7.14 |
| Corrosion Resistance | Excellent, inert to many chemicals | Good, sacrificial anode in galvanization |
| Galvanization Use | Not commonly used due to cost and rarity | Primary choice for protective coating |
| Melting Point (degC) | 3033 | 419.5 |
| Cost | Extremely high | Low and economical |
| Coating Durability | Very high, but impractical | Moderate, effective for rust prevention |
Overview of Galvanization: Purpose and Methods
Galvanization is a process that protects metals from corrosion by applying a protective zinc coating, primarily used in construction and automotive industries to enhance steel durability. Zinc is favored for galvanization due to its excellent sacrificial anode properties, affordability, and ease of application through hot-dip or electroplating methods. Osmium, though a dense and corrosion-resistant metal, is impractical for galvanization because of its high cost, rarity, and poor economic feasibility compared to zinc's widespread use.
Key Properties of Osmium in Metal Applications
Osmium exhibits exceptional hardness, high density, and remarkable corrosion resistance, making it valuable in specialized metal applications where durability under extreme conditions is critical. Its high melting point and robustness against oxidation surpass zinc, which is more commonly utilized for galvanization due to cost-effectiveness and adequate protection against rust in less demanding environments. The rarity and expense of osmium limit its widespread use, but its superior wear resistance and stability make it ideal for niche applications requiring long-lasting performance beyond standard zinc coatings.
Zinc: The Traditional Choice for Galvanization
Zinc remains the traditional and most widely used metal for galvanization due to its excellent corrosion resistance and cost-effectiveness. Its ability to form a protective oxide layer prevents rust and extends the lifespan of steel structures. Despite the emergence of metals like osmium, zinc's proven reliability and abundance make it the preferred choice in industrial galvanization processes.
Corrosion Resistance: Osmium vs Zinc
Osmium exhibits exceptional corrosion resistance due to its high density and inert nature, making it highly durable in harsh environments. Zinc, commonly used in galvanization, provides effective sacrificial protection by corroding preferentially to steel, thereby preventing rust formation. While osmium's corrosion resistance surpasses zinc's, its rarity and cost limit practical use in widespread galvanization applications.
Cost Analysis: Osmium Compared to Zinc
Osmium's high cost, driven by its rarity and extraction complexity, makes it economically unfeasible for galvanization compared to zinc, which is abundant and inexpensive. Zinc offers effective corrosion resistance at a fraction of osmium's market price, making it the industry standard for galvanizing steel. The cost disparity heavily favors zinc, as osmium's premium price outweighs any marginal performance benefits in protective coatings.
Environmental Impact of Osmium and Zinc Galvanization
Osmium, a rare and dense metal, is rarely used in galvanization due to its high toxicity and environmental hazards, posing significant risks during extraction and disposal processes. Zinc galvanization remains the industry standard, offering effective corrosion resistance with a relatively low environmental footprint, especially when sourced sustainably and recycled. Zinc's biodegradability and lower toxicity make it a more environmentally responsible choice compared to osmium for protective metal coatings.
Availability and Sourcing of Osmium vs Zinc
Zinc is widely available and extensively sourced from abundant mineral deposits such as sphalerite, making it the preferred choice for galvanization due to its cost-effectiveness and accessibility. Osmium, a rare platinum-group metal, is extremely scarce and primarily obtained as a byproduct of nickel and platinum mining, resulting in limited availability and significantly higher costs. The constrained sourcing and rarity of osmium make it impractical for galvanization compared to the abundant and economically viable zinc.
Industrial Applications: Practicality in Large-Scale Galvanization
Zinc remains the preferred metal for large-scale galvanization due to its cost-effectiveness, availability, and excellent corrosion resistance, making it ideal for industrial applications such as steel protection in construction and automotive manufacturing. Osmium, despite its exceptional hardness and corrosion resistance, is impractical for galvanization on an industrial scale due to its extreme rarity and high cost, limiting its use to specialized, small-scale applications. The scalability and economic feasibility of zinc galvanization significantly outweigh osmium's superior but niche properties in mass production environments.
Safety and Handling Concerns: Osmium vs Zinc
Osmium and zinc differ significantly in safety and handling for galvanization; zinc is widely used due to its lower toxicity and ease of handling, whereas osmium poses considerable risks, including toxicity and the formation of highly reactive osmium tetroxide. Zinc's comparatively stable and non-toxic nature makes it safer for workers and reduces environmental hazards during galvanization processes. Proper ventilation and protective equipment are critical when handling osmium due to its volatile and toxic compounds, making zinc the preferred choice for industrial galvanization applications where safety is a priority.
Future Trends: Innovations in Galvanization Materials
Emerging innovations in galvanization materials prioritize the use of osmium alloys due to their exceptional corrosion resistance and durability, surpassing traditional zinc coatings. Recent research highlights osmium's potential to enhance protective layers in extreme environments, offering longer lifespan and reduced maintenance costs. Zinc remains widely used for its cost-effectiveness and ease of application, but advancements in nanostructured osmium composites suggest a future shift toward more resilient and sustainable galvanization solutions.
Infographic: Osmium vs Zinc for Galvanization
 azmater.com
azmater.com