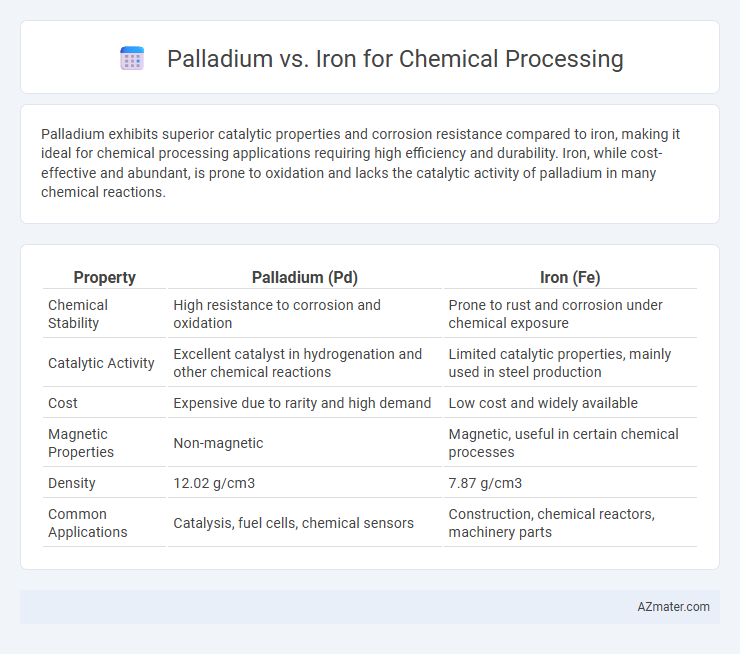
Palladium exhibits superior catalytic properties and corrosion resistance compared to iron, making it ideal for chemical processing applications requiring high efficiency and durability. Iron, while cost-effective and abundant, is prone to oxidation and lacks the catalytic activity of palladium in many chemical reactions.
Table of Comparison
| Property | Palladium (Pd) | Iron (Fe) |
|---|---|---|
| Chemical Stability | High resistance to corrosion and oxidation | Prone to rust and corrosion under chemical exposure |
| Catalytic Activity | Excellent catalyst in hydrogenation and other chemical reactions | Limited catalytic properties, mainly used in steel production |
| Cost | Expensive due to rarity and high demand | Low cost and widely available |
| Magnetic Properties | Non-magnetic | Magnetic, useful in certain chemical processes |
| Density | 12.02 g/cm3 | 7.87 g/cm3 |
| Common Applications | Catalysis, fuel cells, chemical sensors | Construction, chemical reactors, machinery parts |
Introduction to Palladium and Iron in Chemical Processing
Palladium is a rare transition metal widely valued in chemical processing for its exceptional catalytic properties, especially in hydrogenation and carbon-carbon coupling reactions. Iron, a more abundant and cost-effective metal, serves as a versatile catalyst in processes such as ammonia synthesis and Fischer-Tropsch reactions, owing to its strong redox capabilities. The choice between palladium and iron depends on factors like catalytic efficiency, reaction specificity, cost, and sustainability in industrial chemical applications.
Chemical Properties: Palladium vs Iron
Palladium exhibits exceptional catalytic properties and resistance to corrosion, making it highly effective in hydrogenation and catalytic converter applications, while iron is prone to oxidation and less effective as a catalyst in chemical processing. The atomic structure of palladium allows for reversible hydrogen absorption, enhancing its role in hydrogen storage and chemical reactions involving hydrogen transfer. Iron's reactivity and abundance make it suitable for processes like the Haber-Bosch ammonia synthesis, but its chemical instability limits its use in highly corrosive environments compared to palladium.
Catalytic Efficiency Comparison
Palladium exhibits superior catalytic efficiency compared to iron in chemical processing due to its higher activity in hydrogenation and carbon-carbon coupling reactions, resulting in faster reaction rates and greater selectivity. Iron catalysts often require harsher conditions and longer reaction times, reducing overall process efficiency in industrial applications. The enhanced surface properties and electronic structure of palladium facilitate more effective adsorption and activation of reactants, making it the preferred choice for high-precision catalytic processes.
Corrosion Resistance and Durability
Palladium demonstrates superior corrosion resistance compared to iron, especially in highly acidic and oxidative chemical processing environments, due to its noble metal properties that prevent rust and degradation. Iron, while more cost-effective, is prone to corrosion such as rust and oxidation, requiring protective coatings or alloying with chromium to enhance durability. The enhanced longevity of palladium in corrosive settings significantly reduces maintenance needs and equipment downtime, making it a preferred choice for critical chemical processing applications.
Cost and Availability Analysis
Palladium, a rare and costly precious metal, significantly exceeds iron in price, often costing thousands of dollars per ounce compared to iron's abundant and inexpensive availability. Iron's widespread global reserves contribute to its low cost and ease of procurement, making it a preferred material for large-scale chemical processing applications where cost efficiency is crucial. Palladium's scarcity and high demand in catalytic converters and specialized chemical reactions limit its availability, resulting in supply constraints and price volatility.
Environmental Impact of Palladium and Iron
Palladium's environmental impact is primarily associated with its extraction and refining, which involve energy-intensive processes and the release of hazardous chemicals, often leading to soil and water contamination. Iron, although abundant and widely recycled, requires large-scale mining operations that contribute to deforestation, habitat loss, and significant carbon emissions from smelting activities. Both metals present environmental challenges, but iron's higher availability and recycling potential generally result in a lower overall environmental footprint compared to the scarce and more impactful extraction process of palladium.
Common Industrial Applications
Palladium is extensively used in catalytic converters, hydrogenation processes, and fuel cell technology due to its excellent resistance to corrosion and ability to facilitate hydrogen absorption. Iron finds primary applications in chemical processing as a cost-effective catalyst in the Haber-Bosch process for ammonia synthesis and Fischer-Tropsch synthesis for converting syngas into hydrocarbons. The choice between palladium and iron often depends on the desired catalytic efficiency, reaction specificity, and operational cost constraints in industrial-scale chemical production.
Safety and Handling Considerations
Palladium, prized for its catalytic efficiency in chemical processing, requires careful handling due to its high cost and potential for poisoning by contaminants, necessitating clean environments and controlled conditions to maintain activity and prevent degradation. Iron, commonly used for its durability and cost-effectiveness, poses safety concerns related to corrosion and rust, which can introduce impurities and compromise reaction integrity, demanding proper storage and maintenance protocols. Both metals demand tailored safety measures: palladium's sensitivity to contaminants and iron's susceptibility to oxidation must be managed to ensure operational safety and process consistency.
Advances in Palladium and Iron Technologies
Recent advances in palladium technologies have significantly enhanced catalytic efficiency and selectivity in chemical processing, leveraging nanostructured palladium catalysts for improved hydrogenation and carbon-carbon coupling reactions. Iron catalysts have benefited from developments in fine-tuning active sites and support materials, increasing their performance in sustainable oxidation and Fischer-Tropsch synthesis processes. Innovations in alloying palladium with iron have created hybrid catalysts that combine the cost-effectiveness of iron with the superior catalytic properties of palladium, driving more efficient and eco-friendly chemical manufacturing.
Choosing Between Palladium and Iron for Chemical Processing
Palladium offers superior catalytic properties and resistance to corrosion, making it ideal for hydrogenation and catalytic converter applications in chemical processing. Iron, while more cost-effective and abundant, lacks the catalytic efficiency and durability found in palladium, limiting its use to less demanding reactions. Selecting between palladium and iron depends on balancing budget constraints against the need for catalytic performance and operational longevity.
Infographic: Palladium vs Iron for Chemical Processing
 azmater.com
azmater.com