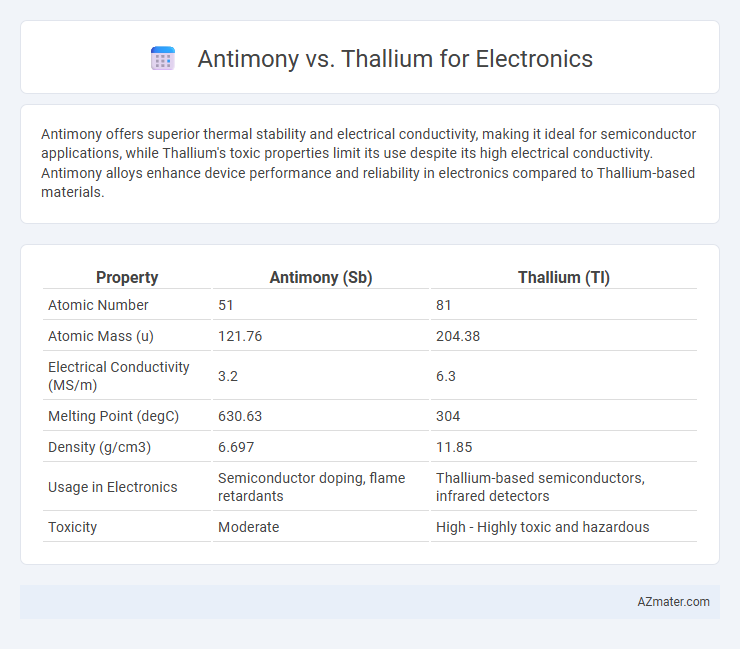
Antimony offers superior thermal stability and electrical conductivity, making it ideal for semiconductor applications, while Thallium's toxic properties limit its use despite its high electrical conductivity. Antimony alloys enhance device performance and reliability in electronics compared to Thallium-based materials.
Table of Comparison
| Property | Antimony (Sb) | Thallium (Tl) |
|---|---|---|
| Atomic Number | 51 | 81 |
| Atomic Mass (u) | 121.76 | 204.38 |
| Electrical Conductivity (MS/m) | 3.2 | 6.3 |
| Melting Point (degC) | 630.63 | 304 |
| Density (g/cm3) | 6.697 | 11.85 |
| Usage in Electronics | Semiconductor doping, flame retardants | Thallium-based semiconductors, infrared detectors |
| Toxicity | Moderate | High - Highly toxic and hazardous |
Introduction to Antimony and Thallium in Electronics
Antimony and thallium are critical elements in electronics due to their unique semiconductor properties, with antimony commonly used in infrared detectors, diodes, and Hall-effect devices. Thallium, although less prevalent, serves in specialized electronic applications such as low-temperature superconductors and certain types of photodetectors. The integration of antimony enhances device performance through its ability to create stable n-type semiconductors, while thallium's role centers on modifying electronic and thermal conductivity in niche technologies.
Elemental Properties: Antimony vs Thallium
Antimony exhibits a higher melting point (630.6degC) compared to thallium (304degC), making it more suitable for applications requiring thermal stability in electronics. Antimony's semimetallic nature and moderate electrical conductivity contrast with thallium's metallic character and higher conductivity, influencing their roles in semiconductor and alloy compositions. The distinct atomic numbers (51 for antimony, 81 for thallium) and electron configurations affect their chemical reactivity and compatibility in electronic materials and device fabrication.
Electrical Conductivity Comparison
Antimony exhibits higher electrical conductivity than thallium due to its semimetallic properties, which make it suitable for use in semiconductors and electronic components such as diodes and infrared detectors. Thallium, with its lower electrical conductivity and higher resistivity, is less favored in electronic applications but is sometimes used in specialized optical devices and low-melting alloys. The conductivity difference is primarily attributed to antimony's crystal structure and electron mobility, making it a more efficient material for current flow in electronic circuits.
Semiconductor Applications: Antimony and Thallium
Antimony plays a crucial role in semiconductor technology, particularly in compound semiconductors like indium antimonide (InSb), which exhibit high electron mobility ideal for infrared detectors and high-speed transistors. Thallium, though less common, is utilized in certain specialized semiconductor alloys to modify bandgap properties and enhance infrared optoelectronic device performance. The superiority of antimony in commercial semiconductor applications is due to its stable integration and efficient electron transport characteristics compared to thallium's niche usage in optoelectronics.
Material Stability and Durability
Antimony exhibits superior material stability and durability compared to thallium when used in electronic applications due to its higher melting point (630.63degC) and resistance to oxidation. Thallium, with a lower melting point of 304degC and greater chemical reactivity, is more prone to degradation under thermal and environmental stress, limiting its lifespan in devices. The robust structural integrity of antimony compounds enhances long-term performance in semiconductors and alloys, making it a more reliable choice for durable electronic components.
Toxicity and Environmental Impact
Antimony and thallium both present significant toxicity concerns in electronic applications, with thallium exhibiting higher acute toxicity and more severe bioaccumulation risks. Antimony compounds tend to persist in the environment, contributing to soil and water contamination, whereas thallium's environmental impact includes high toxicity to aquatic life even at low concentrations. Regulatory agencies limit thallium usage more strictly due to its greater potential for ecological harm and human health hazards during manufacturing and disposal processes.
Cost and Availability in Electronics Manufacturing
Antimony offers greater cost efficiency and widespread availability compared to thallium, making it more favorable in electronics manufacturing. Thallium's rarity and toxic nature result in higher procurement costs and stricter handling regulations, limiting its practical use. Consequently, manufacturers prioritize antimony for applications like semiconductors and flame retardants due to its economic benefits and reliable supply chain.
Recent Advances in Antimony and Thallium Technologies
Recent advances in antimony-based semiconductor technologies have significantly enhanced thermoelectric device efficiency and infrared detector sensitivity. Thallium compounds, particularly thallium-based chalcogenides, have shown promising improvements in nonlinear optical applications and photoconductive performance. Emerging research in antimony-tin oxide and thallium-doped materials focuses on optimizing electronic band structures and carrier mobility for next-generation optoelectronic devices.
Antimony vs Thallium: Suitability for Emerging Electronics
Antimony exhibits superior thermal stability and higher electron mobility compared to thallium, making it more suitable for high-performance semiconductors in emerging electronics such as infrared detectors and power devices. Thallium's toxicity and lower abundance limit its practical applications despite its potential in optoelectronic and thermoelectric components. Antimony's compatibility with existing silicon-based technologies and its ability to enhance device efficiency position it as a preferred choice for sustainable, next-generation electronic materials.
Future Prospects and Research Directions
Antimony and thallium are critical in advancing electronic materials, with antimony showing promise in thermoelectric devices and next-generation semiconductors due to its favorable electronic band structure and low toxicity compared to thallium. Current research focuses on enhancing antimony-based compound semiconductors for flexible electronics and high-speed transistors, while thallium's toxicological challenges limit its widespread use despite its unique semiconductor properties. Future prospects emphasize developing novel antimony alloys and nanoscale structures to optimize performance in optoelectronics and energy-efficient components, positioning antimony as a safer, more sustainable alternative in cutting-edge electronic research.
Infographic: Antimony vs Thallium for Electronic
 azmater.com
azmater.com