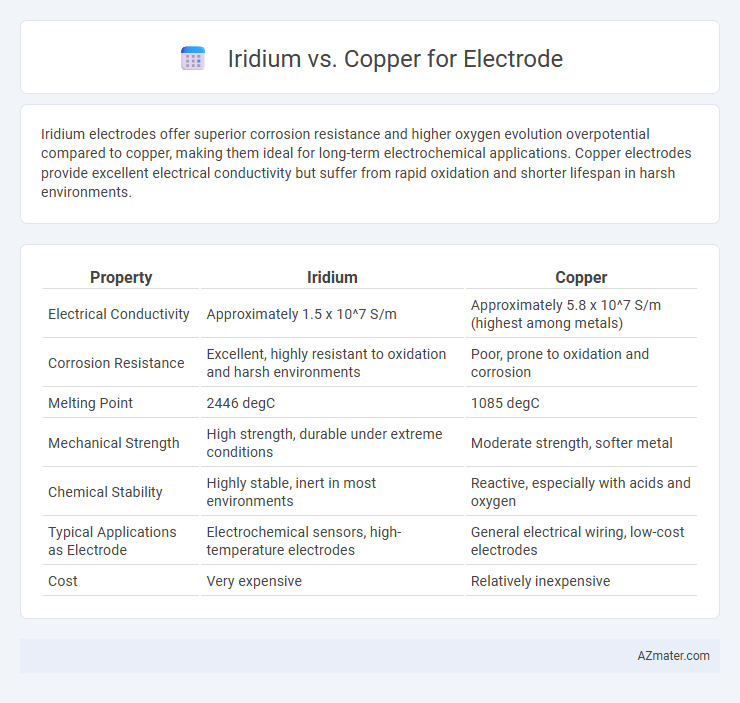
Iridium electrodes offer superior corrosion resistance and higher oxygen evolution overpotential compared to copper, making them ideal for long-term electrochemical applications. Copper electrodes provide excellent electrical conductivity but suffer from rapid oxidation and shorter lifespan in harsh environments.
Table of Comparison
| Property | Iridium | Copper |
|---|---|---|
| Electrical Conductivity | Approximately 1.5 x 10^7 S/m | Approximately 5.8 x 10^7 S/m (highest among metals) |
| Corrosion Resistance | Excellent, highly resistant to oxidation and harsh environments | Poor, prone to oxidation and corrosion |
| Melting Point | 2446 degC | 1085 degC |
| Mechanical Strength | High strength, durable under extreme conditions | Moderate strength, softer metal |
| Chemical Stability | Highly stable, inert in most environments | Reactive, especially with acids and oxygen |
| Typical Applications as Electrode | Electrochemical sensors, high-temperature electrodes | General electrical wiring, low-cost electrodes |
| Cost | Very expensive | Relatively inexpensive |
Introduction to Electrode Materials
Iridium and copper are prominent electrode materials distinguished by their distinct electrical conductivity and corrosion resistance properties. Iridium offers exceptional durability and oxidation resistance, making it ideal for high-temperature and harsh chemical environments, whereas copper excels in electrical conductivity but is more susceptible to corrosion. The choice between iridium and copper electrodes depends on the specific application requirements, balancing conductivity, longevity, and environmental conditions.
Overview of Iridium and Copper Properties
Iridium exhibits exceptional corrosion resistance, high melting point (2446degC), and excellent electrical conductivity, making it ideal for electrodes in harsh environments and high-temperature applications. Copper offers superior electrical conductivity (5.96 x 10^7 S/m) and thermal conductivity but has lower corrosion resistance compared to iridium, limiting its use in aggressive chemical conditions. The choice between iridium and copper electrodes depends on balancing conductivity requirements with environmental durability and cost considerations.
Electrical Conductivity Comparison
Iridium electrodes offer lower electrical conductivity compared to copper, with copper exhibiting a conductivity of approximately 5.96 x 10^7 S/m, making it one of the most efficient conductors. Iridium's conductivity is significantly less, around 1.90 x 10^7 S/m, limiting its use in applications demanding maximum current flow. Despite lower conductivity, iridium provides superior corrosion resistance and durability, often preferred in harsh chemical environments where copper electrodes might degrade.
Corrosion Resistance: Iridium vs Copper
Iridium exhibits superior corrosion resistance compared to copper, maintaining structural integrity in harsh chemical environments such as strong acids and salts. Copper tends to corrode rapidly when exposed to moisture, oxygen, and electrolytes, leading to surface degradation and reduced electrical performance. The high corrosion resistance of iridium makes it a preferred material for electrodes in industrial applications requiring long-term durability and stability.
Electrochemical Performance and Efficiency
Iridium electrodes exhibit superior electrochemical performance compared to copper due to their higher catalytic activity and exceptional corrosion resistance in harsh environments, resulting in enhanced stability and longer lifespan during electrolysis processes. Copper electrodes, while cost-effective and highly conductive, tend to suffer from rapid oxidation and degradation, leading to reduced efficiency and frequent replacement. The efficiency of iridium electrodes in oxygen evolution reactions (OER) far surpasses that of copper, making iridium the preferred choice for high-performance energy conversion and water-splitting applications.
Longevity and Durability in Applications
Iridium electrodes exhibit superior longevity and durability compared to copper due to iridium's exceptional resistance to corrosion, high melting point, and stability in harsh chemical environments. Copper electrodes tend to degrade faster under high temperatures and oxidative conditions, leading to reduced lifespan in demanding applications. Iridium's robustness ensures consistent electrical performance and reliability, making it the preferred choice for long-term, high-stress electrochemical systems.
Cost Analysis of Iridium and Copper Electrodes
Iridium electrodes exhibit significantly higher costs compared to copper electrodes due to iridium's rarity and complex extraction processes, with prices often exceeding $4,500 per ounce versus copper's $4-$5 per pound. The investment in iridium electrodes is justified in high-performance applications requiring superior corrosion resistance and conductivity despite the elevated initial expenses. Copper electrodes offer cost-effective solutions for standard electrical applications, providing a balance between performance and affordability, making them suitable for large-scale industrial uses where budget constraints are critical.
Environmental and Safety Considerations
Iridium electrodes offer superior corrosion resistance and biocompatibility compared to copper, reducing environmental contamination risks from metal leaching. Copper electrodes pose higher toxicity concerns and potential environmental hazards due to copper ion release during electrochemical processes. Safety considerations favor iridium for applications requiring stability and long-term use, as copper may degrade and cause electrical instability or toxic exposure.
Typical Applications: Where Each Excels
Iridium electrodes excel in high-temperature and corrosive environments such as electrochemical sensors, fuel cells, and industrial electrolysis due to their exceptional corrosion resistance and stability. Copper electrodes are preferable for applications requiring excellent electrical conductivity and cost efficiency, including battery terminals, electrical wiring, and low-voltage contact points. In summary, iridium suits demanding, long-life applications while copper is ideal for general electrical and electronic uses.
Choosing the Best Electrode: Key Factors
Iridium electrodes exhibit superior corrosion resistance and higher conductivity, making them ideal for demanding electrochemical applications compared to copper electrodes, which are more prone to oxidation and wear. When choosing the best electrode, critical factors include electrical conductivity, durability under operational conditions, and cost-effectiveness; iridium's long lifespan and stability often justify its premium price. Copper electrodes provide excellent initial conductivity and affordability but may require frequent replacement in harsh environments, whereas iridium ensures consistent performance in precision and high-current settings.
Infographic: Iridium vs Copper for Electrode
 azmater.com
azmater.com