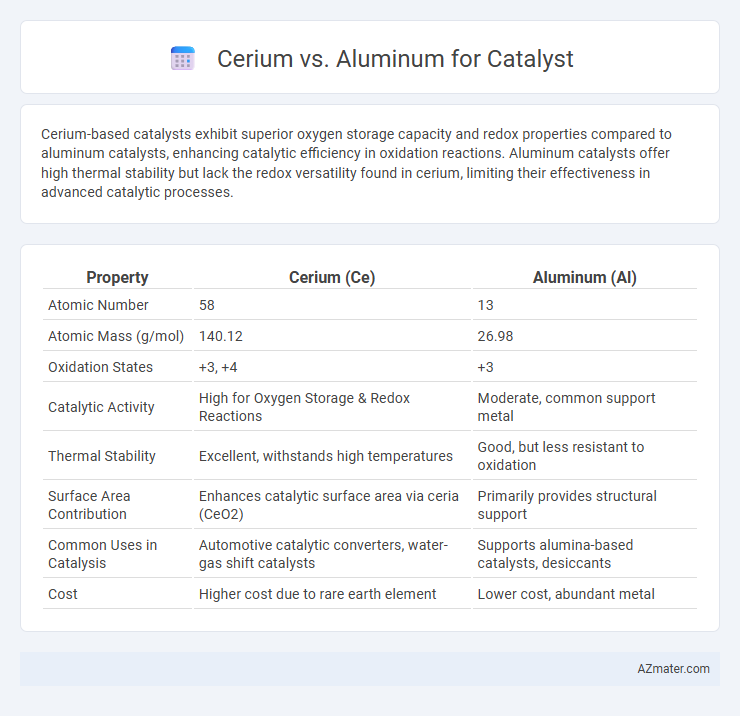
Cerium-based catalysts exhibit superior oxygen storage capacity and redox properties compared to aluminum catalysts, enhancing catalytic efficiency in oxidation reactions. Aluminum catalysts offer high thermal stability but lack the redox versatility found in cerium, limiting their effectiveness in advanced catalytic processes.
Table of Comparison
| Property | Cerium (Ce) | Aluminum (Al) |
|---|---|---|
| Atomic Number | 58 | 13 |
| Atomic Mass (g/mol) | 140.12 | 26.98 |
| Oxidation States | +3, +4 | +3 |
| Catalytic Activity | High for Oxygen Storage & Redox Reactions | Moderate, common support metal |
| Thermal Stability | Excellent, withstands high temperatures | Good, but less resistant to oxidation |
| Surface Area Contribution | Enhances catalytic surface area via ceria (CeO2) | Primarily provides structural support |
| Common Uses in Catalysis | Automotive catalytic converters, water-gas shift catalysts | Supports alumina-based catalysts, desiccants |
| Cost | Higher cost due to rare earth element | Lower cost, abundant metal |
Introduction to Cerium and Aluminum as Catalysts
Cerium, a rare earth metal, is widely used in catalysts for its excellent oxygen storage capacity and redox properties, making it effective in automotive catalytic converters and oxidation reactions. Aluminum, primarily in the form of alumina (Al2O3), serves as a catalyst support due to its high surface area, thermal stability, and ability to disperse active catalytic species effectively. Both metals enhance catalytic performance but cerium often plays an active role in redox reactions, whereas aluminum mainly provides structural support in catalytic systems.
Chemical Properties Relevant to Catalysis
Cerium exhibits variable oxidation states (Ce3+ and Ce4+) that facilitate oxygen storage and release, enhancing redox reactions essential for catalytic processes. Aluminum primarily exists in the +3 oxidation state and acts as a stable support material due to its high surface area and thermal stability but lacks intrinsic redox activity. The unique ability of cerium oxide (ceria) to undergo reversible Ce3+/Ce4+ transitions makes it superior in oxygen transfer reactions compared to aluminum oxide, influencing catalyst efficiency and durability.
Catalyst Performance: Cerium vs Aluminum
Cerium outperforms aluminum in catalyst performance due to its superior oxygen storage capacity and redox properties, enabling enhanced catalytic activity and durability in oxidation and reduction reactions. Cerium-based catalysts exhibit higher resistance to poisoning and sintering compared to aluminum oxide supports, resulting in sustained efficiency in automotive catalytic converters and industrial processes. The unique Ce3+/Ce4+ redox cycle facilitates rapid oxygen release and uptake, significantly accelerating reaction rates beyond what aluminum oxide catalysts can achieve.
Redox Behavior and Oxygen Storage Capacity
Cerium exhibits superior redox behavior compared to aluminum due to its ability to easily switch between Ce3+ and Ce4+ oxidation states, enhancing its catalytic efficiency in oxidation-reduction reactions. The oxygen storage capacity (OSC) of cerium oxide (ceria) significantly surpasses that of aluminum oxide, allowing it to reversibly release and store oxygen under fluctuating reaction conditions. This dynamic oxygen buffering capability makes cerium-based catalysts highly effective for applications such as automotive exhaust treatment and oxidative catalysis, where oxygen mobility is critical.
Thermal Stability and Catalyst Lifespan
Cerium exhibits superior thermal stability compared to aluminum when used in catalysts, maintaining structural integrity at temperatures exceeding 800degC, whereas aluminum tends to degrade or sinter above 600degC. This enhanced thermal resistance of cerium-based catalysts results in prolonged catalyst lifespan, reducing deactivation rates in high-temperature reactions such as automotive exhaust treatment and industrial oxidation processes. The ability of cerium to store and release oxygen efficiently also contributes to sustained catalytic performance under thermal stress, making it a preferred choice for applications demanding long-term durability.
Application Areas: Automotive to Industrial Processes
Cerium-based catalysts excel in automotive applications by enhancing emissions control through improved oxygen storage capacity, crucial for three-way catalytic converters in gasoline engines and diesel particulate filters. Aluminum catalysts are preferred in industrial processes such as petrochemical refining and dehydration reactions due to their high thermal stability and acid-site density, facilitating hydrocarbon cracking and synthesis. Combining cerium and aluminum oxides in catalyst formulations tailors performance for both mobile emission reduction and stationary industrial chemical transformations.
Environmental Impact and Sustainability
Cerium-based catalysts outperform aluminum catalysts in environmental impact due to their superior catalytic efficiency in reducing harmful emissions from automotive exhaust systems, notably decreasing nitrogen oxides (NOx) and carbon monoxide (CO) levels. Cerium's abundance and recyclability contribute to greater sustainability, whereas aluminum production demands significant energy and results in higher greenhouse gas emissions. Selecting cerium catalysts supports eco-friendly industrial processes by enhancing air quality and minimizing resource depletion.
Cost-Effectiveness and Market Availability
Cerium offers superior catalytic performance in oxidation reactions due to its high oxygen storage capacity, making it more cost-effective in long-term applications despite a higher initial price compared to aluminum. Aluminum is abundant and inexpensive, which ensures wide market availability and lower upfront costs but lacks the catalytic efficiency and durability of cerium-based catalysts. The balance between upfront material costs and catalytic efficiency favors cerium in industrial catalytic converters, especially in automotive and environmental sectors.
Challenges and Limitations in Catalyst Use
Cerium catalysts face challenges such as high cost and sensitivity to moisture, which can reduce their catalytic efficiency and lifespan. Aluminum-based catalysts, while more economical and abundant, often suffer from lower activity and stability under harsh reaction conditions, limiting their applicability. Both materials require advanced engineering to overcome issues like sintering and deactivation for sustained catalytic performance.
Future Trends in Catalyst Development
Cerium's superior oxygen storage capacity and redox properties position it as a critical material in next-generation catalyst development, especially for automotive catalytic converters and environmental applications. Aluminum, while valued for its lightweight and structural support roles, primarily serves as a substrate or stabilizer rather than an active catalytic agent. Future trends emphasize cerium-based mixed oxides and nanostructures to enhance catalytic efficiency, durability, and emissions reduction, reflecting a shift towards more sustainable and high-performance catalyst materials.
Infographic: Cerium vs Aluminum for Catalyst
 azmater.com
azmater.com