Copper offers superior electrical conductivity and cost-effectiveness for battery anodes, while nickel provides higher energy density and improved thermal stability in cathode materials. Choosing copper enhances charge efficiency, whereas nickel boosts battery lifespan and performance under high-stress conditions.
Table of Comparison
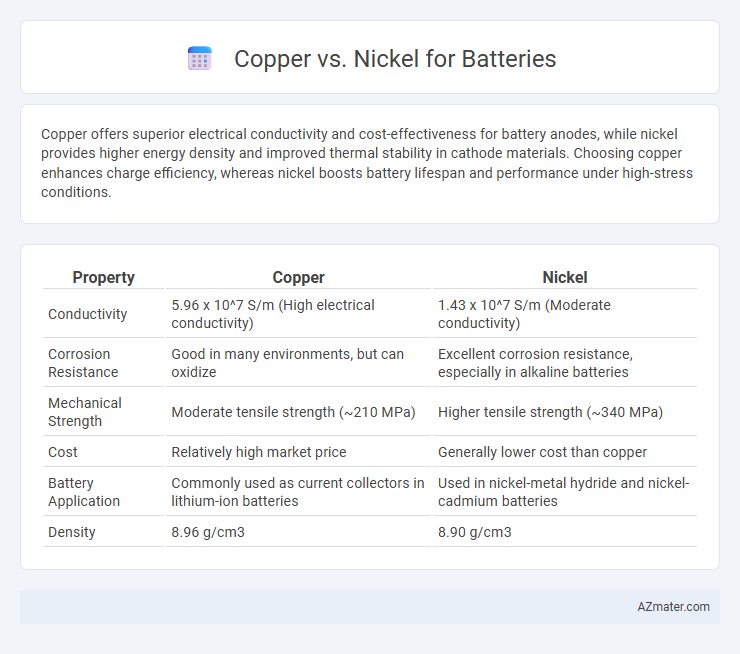
| Property | Copper | Nickel |
|---|---|---|
| Conductivity | 5.96 x 10^7 S/m (High electrical conductivity) | 1.43 x 10^7 S/m (Moderate conductivity) |
| Corrosion Resistance | Good in many environments, but can oxidize | Excellent corrosion resistance, especially in alkaline batteries |
| Mechanical Strength | Moderate tensile strength (~210 MPa) | Higher tensile strength (~340 MPa) |
| Cost | Relatively high market price | Generally lower cost than copper |
| Battery Application | Commonly used as current collectors in lithium-ion batteries | Used in nickel-metal hydride and nickel-cadmium batteries |
| Density | 8.96 g/cm3 | 8.90 g/cm3 |
Introduction: Copper vs Nickel in Battery Technology
Copper and nickel are critical metals in battery technology, each playing a distinct role in enhancing performance and durability. Copper primarily serves as the anode current collector due to its excellent electrical conductivity and corrosion resistance, enabling efficient electron flow. Nickel is commonly used in cathode materials, such as nickel-cobalt-manganese (NCM) or nickel-cobalt-aluminum (NCA) chemistries, because it improves energy density and cycle life in lithium-ion batteries.
Material Properties: Copper and Nickel Comparison
Copper exhibits excellent electrical conductivity of approximately 5.96 x 10^7 S/m, making it highly efficient for current collection in batteries. Nickel offers superior corrosion resistance and maintains structural integrity at elevated temperatures, crucial for battery longevity and safety. The mechanical strength of nickel surpasses copper, providing durability, while copper's thermal conductivity enhances heat dissipation during battery operation.
Electrical Conductivity and Performance
Copper exhibits superior electrical conductivity compared to nickel, with a conductivity of approximately 59.6 MS/m versus nickel's 14.3 MS/m, making copper the preferred choice for efficient current collection in battery electrodes. Copper's higher conductivity reduces energy losses and heat generation, enhancing overall battery performance and lifespan. In contrast, nickel's lower conductivity is often offset by its corrosion resistance and mechanical strength, but it generally results in higher internal resistance in battery applications.
Role in Battery Chemistry and Design
Copper is widely used as the current collector in lithium-ion batteries due to its excellent electrical conductivity and stability at the negative electrode, facilitating efficient electron flow and minimizing resistance. Nickel serves as a key component in cathode materials, such as nickel-rich layered oxides (NMC and NCA), enhancing energy density and thermal stability through improved electrochemical performance. The combination of copper current collectors with nickel-based cathodes optimizes battery design by balancing conductivity, capacity, and cycle life in high-performance energy storage systems.
Cost Analysis: Copper vs Nickel
Copper offers a lower cost per kilogram compared to nickel, making it a more economical choice for battery production, especially in large-scale applications. Nickel prices tend to be higher due to its stronger demand in high-performance lithium-ion batteries and limited supply, impacting overall material costs. The cost efficiency of copper helps reduce battery manufacturing expenses, but nickel's superior energy density contribution often justifies its premium price in advanced battery chemistries.
Environmental Impact and Sustainability
Copper and nickel are essential metals in battery production, with copper primarily used for electrical wiring and nickel enhancing energy density in lithium-ion batteries. Copper mining has a significant environmental footprint due to habitat destruction, water pollution, and high energy consumption, while nickel extraction often involves toxic sulfur emissions and substantial carbon output. Sustainable battery development demands improved recycling processes for both metals to minimize resource depletion and reduce greenhouse gas emissions associated with mining and processing.
Supply Chain and Availability
Copper remains a critical material in battery supply chains due to its high electrical conductivity and extensive use in electric vehicle (EV) wiring and battery anodes, with global reserves concentrated in Chile and Peru ensuring relatively stable availability. Nickel, essential for high-energy-density batteries like NMC and NCA cathodes, faces supply chain volatility caused by geopolitical risks and ethical concerns in major mining regions such as Indonesia, the Philippines, and Russia. The growing demand for nickel in lithium-ion batteries intensifies pressure on its supply chain, whereas copper benefits from diversified sources and recycling infrastructure that enhance its long-term availability.
Innovations and Emerging Technologies
Copper's superior electrical conductivity and cost-effectiveness drive its increased use in advanced battery current collectors and connectors. Emerging technologies leverage nickel's high energy density and thermal stability to enhance lithium-ion battery cathodes, boosting performance and lifespan. Innovations in copper-nickel alloys optimize both conductivity and corrosion resistance, improving battery efficiency and durability in electric vehicles and grid storage.
Applications in Various Battery Types
Copper is widely used as the current collector in lithium-ion batteries due to its excellent electrical conductivity and resistance to corrosion, particularly in the anodes of graphite-based batteries. Nickel plays a crucial role in cathode materials such as nickel-cobalt-manganese (NCM) and nickel-cobalt-aluminum (NCA) batteries, where it enhances energy density and cycle life, especially in electric vehicle applications. Choosing copper or nickel depends on the battery type and desired performance characteristics, with copper favored for anode current collectors and nickel integral to high-capacity cathodes.
Future Trends in Battery Materials: Copper or Nickel?
Copper and nickel remain critical materials in battery technology, with nickel gaining traction for its higher energy density and improved performance in lithium-ion batteries. Future trends indicate increased nickel usage in cathodes to enhance battery capacity and reduce cobalt dependence, while copper continues to be essential for anode current collectors and electrical conductivity. Innovations in nickel-based cathode chemistries and sustainable copper recycling are shaping a balanced approach to optimize cost, efficiency, and resource availability in next-generation batteries.
Infographic: Copper vs Nickel for Battery
 azmater.com
azmater.com