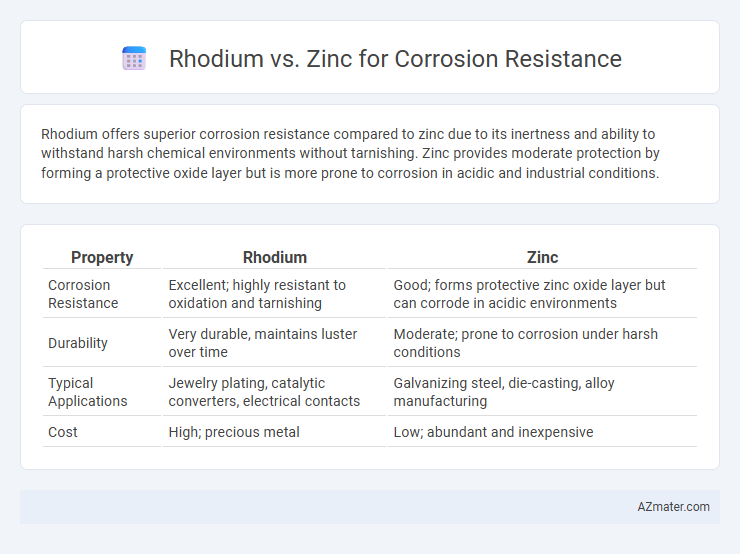
Rhodium offers superior corrosion resistance compared to zinc due to its inertness and ability to withstand harsh chemical environments without tarnishing. Zinc provides moderate protection by forming a protective oxide layer but is more prone to corrosion in acidic and industrial conditions.
Table of Comparison
| Property | Rhodium | Zinc |
|---|---|---|
| Corrosion Resistance | Excellent; highly resistant to oxidation and tarnishing | Good; forms protective zinc oxide layer but can corrode in acidic environments |
| Durability | Very durable, maintains luster over time | Moderate; prone to corrosion under harsh conditions |
| Typical Applications | Jewelry plating, catalytic converters, electrical contacts | Galvanizing steel, die-casting, alloy manufacturing |
| Cost | High; precious metal | Low; abundant and inexpensive |
Introduction to Corrosion Resistance
Rhodium exhibits superior corrosion resistance due to its dense, stable oxide layer that protects against oxidation and chemical attack in harsh environments. Zinc provides effective corrosion resistance primarily through galvanization, forming a sacrificial zinc oxide coating that prevents rust formation on steel surfaces. The choice between rhodium and zinc depends on application-specific requirements such as durability, environmental exposure, and cost constraints.
Overview of Rhodium and Zinc as Metals
Rhodium, a rare platinum-group metal, exhibits exceptional corrosion resistance due to its inert surface and high chemical stability, making it ideal for protective coatings and catalytic applications. Zinc, a more abundant and cost-effective metal, provides effective corrosion resistance primarily through its sacrificial anodic properties, widely used in galvanization to protect steel structures from rust. While rhodium offers superior durability and less reactivity, zinc's practical applications emphasize large-scale protection in industrial and construction environments.
Chemical Properties Affecting Corrosion Resistance
Rhodium exhibits exceptional corrosion resistance due to its stable electron configuration and high electronegativity, which minimize oxidation and chemical reactions with environmental agents. Zinc, with lower electronegativity and a more reactive surface, forms a protective oxide layer that effectively prevents further corrosion, especially in atmospheric conditions. The noble character of rhodium means it resists acidic and oxidative environments better than zinc, whose sacrificial anode properties provide galvanic protection, but reduce its long-term corrosion resistance in harsh chemicals.
Mechanisms of Corrosion Protection: Rhodium vs Zinc
Rhodium offers superior corrosion resistance through its dense, inert oxide layer that prevents oxidation and chemical attack on underlying metals, making it highly effective in harsh environments. Zinc protects through a sacrificial anode mechanism, where it preferentially corrodes, forming a stable zinc oxide or carbonate layer that shields steel substrates from rust. While rhodium's protective barrier is mainly passive and highly durable, zinc's electrochemical protection is active but can degrade over time as the sacrificial coating is consumed.
Application Methods: Plating and Coating Techniques
Rhodium offers superior corrosion resistance through electroplating, providing a hard, reflective surface ideal for jewelry and electronic components. Zinc typically utilizes hot-dip galvanizing or electroplating, forming a sacrificial layer that protects steel from rust in construction and automotive industries. Both metals require precise surface preparation and thickness control to maximize protective benefits in their respective coating applications.
Durability and Longevity in Harsh Environments
Rhodium exhibits superior corrosion resistance compared to zinc due to its noble metal properties and high chemical inertness, making it exceptionally durable in harsh environments such as marine or industrial settings. Zinc serves primarily as a sacrificial anode in galvanization, protecting base metals by corroding preferentially but offering less intrinsic longevity under extreme conditions. The choice between rhodium and zinc coatings depends on balancing cost-effectiveness with the required lifespan and environmental exposure, with rhodium favored for critical applications demanding maximum durability and longevity.
Cost-Efficiency Analysis: Rhodium vs Zinc
Rhodium offers superior corrosion resistance with exceptional durability in harsh environments but comes at significantly higher material and application costs compared to zinc. Zinc provides cost-efficient corrosion protection primarily through sacrificial anode action, making it economically viable for large-scale and industrial uses despite its comparatively shorter lifespan. Evaluating the balance between upfront investment and long-term maintenance expenses is crucial when choosing rhodium or zinc for corrosion resistance solutions.
Common Industrial Applications
Rhodium offers superior corrosion resistance compared to zinc, making it ideal for high-performance industrial applications like catalytic converters and electrical contacts where durability in harsh chemical environments is crucial. Zinc is widely used for galvanizing steel and iron to provide a cost-effective, moderate corrosion barrier in construction, automotive, and marine industries. Industries favor rhodium plating for components requiring extreme wear and oxidation resistance, while zinc remains preferred for large-scale protective coatings due to its affordability and sacrificial anode properties.
Environmental Impact and Sustainability
Rhodium offers superior corrosion resistance with minimal environmental degradation due to its inert nature, making it highly sustainable for long-term applications despite its rarity and high extraction impact. Zinc provides effective corrosion protection through sacrificial anodic behavior, but its mining and refining processes can release significant pollutants, raising concerns about environmental sustainability. Choosing between rhodium and zinc depends on balancing the ecological footprint of metal extraction against the longevity and environmental benefits of enhanced corrosion resistance.
Conclusion: Choosing the Right Metal for Corrosion Resistance
Rhodium offers superior corrosion resistance due to its exceptional inertness and resistance to oxidation, making it ideal for harsh chemical environments. Zinc provides effective sacrificial protection by corroding preferentially to steel, which is beneficial for galvanization applications. Selecting the right metal depends on specific use cases: rhodium suits high-end, long-lasting protective coatings, while zinc is preferred for economical, large-scale rust prevention.
Infographic: Rhodium vs Zinc for Corrosion Resistance
 azmater.com
azmater.com