Stainless steel exhibits high corrosion resistance and cost-efficiency, making it suitable for general chemical catalysis, while platinum offers superior catalytic activity and selectivity in reactions such as hydrogenation and oxidation. Platinum's resistance to poisoning and high durability under harsh reaction conditions justifies its premium use in precision catalytic processes despite higher costs.
Table of Comparison
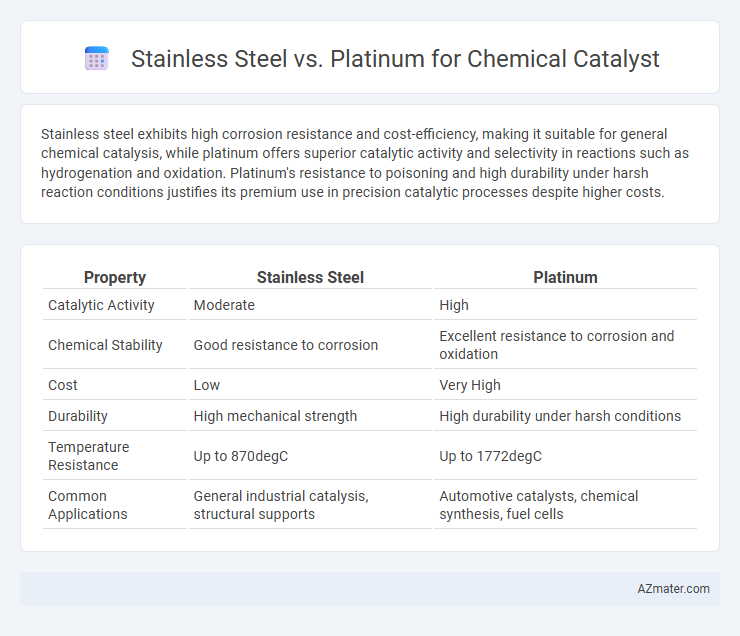
| Property | Stainless Steel | Platinum |
|---|---|---|
| Catalytic Activity | Moderate | High |
| Chemical Stability | Good resistance to corrosion | Excellent resistance to corrosion and oxidation |
| Cost | Low | Very High |
| Durability | High mechanical strength | High durability under harsh conditions |
| Temperature Resistance | Up to 870degC | Up to 1772degC |
| Common Applications | General industrial catalysis, structural supports | Automotive catalysts, chemical synthesis, fuel cells |
Introduction: The Role of Chemical Catalysts
Chemical catalysts accelerate reaction rates by lowering activation energy, playing a crucial role in industrial processes such as hydrogenation and oxidation. Stainless steel, valued for its corrosion resistance and mechanical strength, offers a durable and cost-effective support for catalytic materials under harsh reaction conditions. Platinum, renowned for its superior catalytic activity and selectivity, is widely used in precision applications despite its higher cost and susceptibility to poisoning in certain environments.
Overview of Stainless Steel as a Catalyst Material
Stainless steel is widely used as a catalyst material due to its excellent corrosion resistance, mechanical strength, and cost-effectiveness, making it suitable for various chemical reactions. Its surface can be modified or coated with active metals to enhance catalytic activity, particularly in hydrogenation and oxidation processes. Compared to platinum, stainless steel offers greater durability under harsh reaction conditions, although it generally exhibits lower intrinsic catalytic performance.
Platinum’s Unique Properties in Catalysis
Platinum exhibits exceptional catalytic properties due to its high chemical stability, corrosion resistance, and excellent ability to adsorb and activate a wide range of chemical species. Unlike stainless steel, platinum's unique electron configuration and surface energy enable enhanced catalytic activity and selectivity in reactions such as hydrogenation, oxidation, and reforming processes. Its superior tolerance to harsh chemical environments and resistance to poisoning make platinum indispensable in industrial catalysis applications.
Chemical Resistance: Stainless Steel vs. Platinum
Platinum exhibits superior chemical resistance compared to stainless steel, maintaining stability in highly corrosive environments and strong acids, which is critical for catalytic applications. Stainless steel, though cost-effective and durable, is prone to corrosion and pitting when exposed to aggressive chemicals or high-temperature acidic conditions. The inherent inertness of platinum makes it the preferred choice for catalysts in harsh chemical processes where long-term resistance and performance are essential.
Catalytic Efficiency and Reactivity Comparison
Platinum exhibits superior catalytic efficiency and reactivity compared to stainless steel due to its higher surface energy and ability to adsorb reactant molecules more effectively, facilitating faster reaction rates in chemical processes. Stainless steel, while durable and cost-effective, generally shows lower catalytic activity and may require surface modifications or coatings to enhance its catalytic properties. The inherent electronic structure of platinum allows it to catalyze a broader range of reactions with greater selectivity and stability under harsh chemical conditions.
Cost-Effectiveness and Economic Considerations
Stainless steel offers a lower initial cost and greater mechanical durability compared to platinum, making it a cost-effective choice for large-scale chemical catalysts where budget constraints are critical. Platinum provides superior catalytic activity and resistance to corrosion, which translates to longer lifetime and reduced replacement frequency in high-demand applications, justifying its higher upfront expense. Economic considerations favor stainless steel in processes with moderate catalytic requirements, whereas platinum is preferred for highly specialized or precision chemical reactions that demand exceptional catalyst performance.
Durability and Longevity in Industrial Applications
Stainless steel offers high corrosion resistance and mechanical strength, making it durable for a wide range of industrial chemical catalyst applications, especially in harsh environments with varying temperatures and pressures. Platinum, while significantly more expensive, provides exceptional catalytic efficiency and unmatched resistance to chemical degradation and oxidation, resulting in superior longevity under extreme operating conditions. Industrial catalysts often balance cost and performance by selecting stainless steel for general applications and platinum for specialized processes requiring prolonged catalyst lifespan and optimal reactivity.
Environmental Impact and Sustainability
Stainless steel catalysts offer high durability and corrosion resistance, making them reusable and minimizing environmental waste over multiple reaction cycles. Platinum catalysts, though highly efficient and selective, pose sustainability challenges due to their rarity, high extraction energy, and associated ecological footprint. Recycling and recovery of platinum from spent catalysts are essential to mitigate environmental impact, whereas stainless steel production benefits from widespread availability and lower resource depletion.
Common Industrial Uses: Stainless Steel vs. Platinum
Stainless steel is widely used in chemical reactors, heat exchangers, and piping systems due to its corrosion resistance, mechanical strength, and cost-effectiveness in industrial applications. Platinum serves as a superior catalyst in catalytic converters, hydrogenation reactions, and fuel cells because of its excellent catalytic activity and resistance to deactivation. Industries prefer stainless steel for structural components, while platinum is favored for high-performance catalytic processes requiring precise chemical conversions.
Conclusion: Choosing the Optimal Catalyst Material
Stainless steel offers cost-efficiency and mechanical durability but lacks the superior corrosion resistance and catalytic activity of platinum. Platinum, while significantly more expensive, provides exceptional chemical inertness and high catalytic efficiency under diverse reaction conditions. Selecting the optimal catalyst material depends on balancing budget constraints, required catalyst lifespan, and specific chemical process demands, with platinum favored for high-performance applications and stainless steel suited for less aggressive environments.
Infographic: Stainless steel vs Platinum for Chemical catalyst
 azmater.com
azmater.com