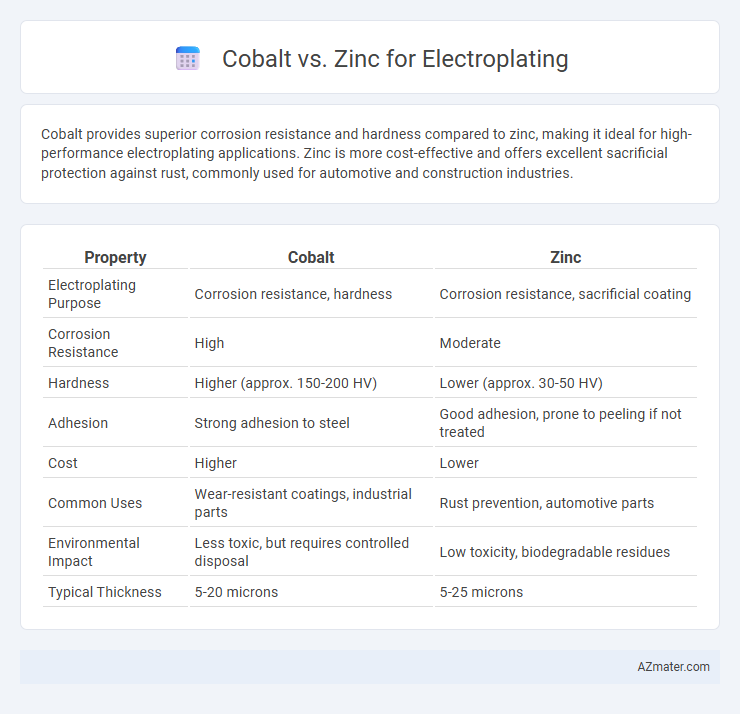
Cobalt provides superior corrosion resistance and hardness compared to zinc, making it ideal for high-performance electroplating applications. Zinc is more cost-effective and offers excellent sacrificial protection against rust, commonly used for automotive and construction industries.
Table of Comparison
| Property | Cobalt | Zinc |
|---|---|---|
| Electroplating Purpose | Corrosion resistance, hardness | Corrosion resistance, sacrificial coating |
| Corrosion Resistance | High | Moderate |
| Hardness | Higher (approx. 150-200 HV) | Lower (approx. 30-50 HV) |
| Adhesion | Strong adhesion to steel | Good adhesion, prone to peeling if not treated |
| Cost | Higher | Lower |
| Common Uses | Wear-resistant coatings, industrial parts | Rust prevention, automotive parts |
| Environmental Impact | Less toxic, but requires controlled disposal | Low toxicity, biodegradable residues |
| Typical Thickness | 5-20 microns | 5-25 microns |
Introduction to Electroplating Metals
Cobalt and zinc are widely used metals in electroplating due to their distinct protective properties and corrosion resistance. Zinc provides excellent sacrificial protection for steel, commonly employed in automotive and construction industries, while cobalt offers superior hardness and wear resistance, suitable for industrial tools and aerospace components. Understanding the electrochemical characteristics of cobalt and zinc is crucial for optimizing coating performance and durability in various applications.
Overview of Cobalt and Zinc in Electroplating
Cobalt and zinc are essential metals used in electroplating to enhance corrosion resistance and surface hardness of substrates. Cobalt electroplating offers superior wear resistance and is commonly employed in aerospace and automotive industries due to its high temperature stability. Zinc plating provides cost-effective corrosion protection, frequently utilized in construction and manufacturing sectors to prevent rust on steel components.
Chemical Properties: Cobalt vs Zinc
Cobalt exhibits superior corrosion resistance and hardness compared to zinc, making it ideal for protective electroplating in harsh environments. Zinc offers excellent sacrificial anode properties with high electrochemical activity, ensuring effective corrosion prevention through galvanic protection. Both metals provide distinct chemical advantages, with cobalt's stability and zinc's reactivity tailored to specific electroplating applications.
Electroplating Process Differences
Cobalt electroplating offers superior hardness and corrosion resistance compared to zinc, making it ideal for wear-resistant applications, while zinc plating excels in cost-effective corrosion protection for steel parts. The electroplating process of cobalt requires an acidic bath with precise pH control, typically using cobalt sulfate or cobalt chloride, whereas zinc plating uses an alkaline or acidic bath with zinc salts like zinc sulfate or zinc chloride. Cobalt plating demands higher current densities and temperature management for optimal deposition quality, whereas zinc plating operates at lower currents and temperatures, simplifying process control and reducing operational costs.
Corrosion Resistance Comparison
Cobalt electroplating offers superior corrosion resistance compared to zinc, particularly in harsh environments due to its dense and hard coating structure. Zinc provides effective sacrificial protection by corroding preferentially, but it is less durable in acidic or marine conditions compared to cobalt. The enhanced corrosion resistance of cobalt makes it ideal for applications requiring long-lasting surface protection and wear resistance.
Surface Finish and Appearance
Cobalt electroplating delivers a hard, bright, and lustrous surface finish with excellent corrosion resistance, making it ideal for decorative and protective coatings requiring a smooth and reflective appearance. Zinc electroplating provides a less shiny, matte or dull surface finish but offers superior sacrificial protection against rust, typically used for industrial applications where corrosion resistance is prioritized over aesthetics. The choice between cobalt and zinc for electroplating depends on whether a high-gloss, visually appealing finish or enhanced corrosion protection with a more utilitarian appearance is desired.
Adhesion Strength and Durability
Cobalt electroplating offers superior adhesion strength compared to zinc, providing enhanced resistance to peeling and flaking in harsh environments. Zinc plating, while cost-effective and corrosion-resistant through passivation, generally exhibits lower durability under mechanical stress and prolonged exposure. The choice between cobalt and zinc for electroplating depends on the application's requirement for long-term wear resistance and adhesion performance.
Environmental and Safety Considerations
Cobalt electroplating poses greater environmental and health risks due to its toxic dust and potential carcinogenic properties, requiring strict handling and disposal measures compared to zinc. Zinc electroplating is generally considered safer and more environmentally friendly, with lower toxicity and easier waste management. However, both metals demand adherence to regulatory guidelines to minimize ecological impact and ensure worker safety.
Cost and Availability of Cobalt and Zinc
Cobalt electroplating typically incurs higher costs due to the metal's scarcity and complex extraction process compared to zinc, which is more abundant and economically accessible. Zinc's widespread availability in the Earth's crust leads to lower raw material prices, making zinc electroplating a cost-effective choice for large-scale applications. The limited global cobalt supply, often concentrated in politically sensitive regions, further elevates its price and affects consistent availability for industrial use.
Choosing the Right Metal for Your Application
Selecting between cobalt and zinc for electroplating hinges on the desired properties and application requirements. Cobalt offers superior hardness, corrosion resistance, and magnetic properties ideal for wear-resistant coatings in automotive and aerospace industries. Zinc provides excellent sacrificial protection against rust at a lower cost, making it suitable for general-purpose coatings on steel or iron components exposed to moderate environmental conditions.
Infographic: Cobalt vs Zinc for Electroplating
 azmater.com
azmater.com