Antimony is a non-toxic metalloid used as a flame retardant and in semiconductor components for energy-efficient lighting. Thorium, a radioactive actinide, was historically used in gas mantles for bright light but is now largely replaced due to health risks and disposal challenges.
Table of Comparison
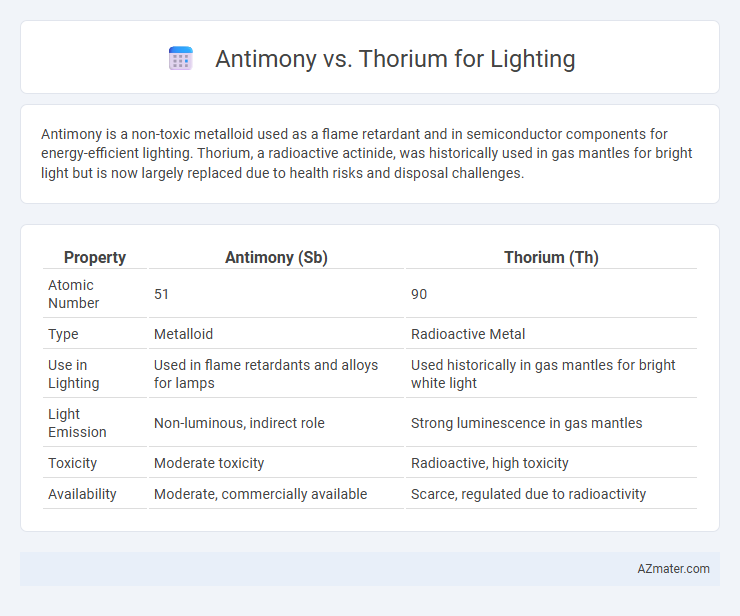
| Property | Antimony (Sb) | Thorium (Th) |
|---|---|---|
| Atomic Number | 51 | 90 |
| Type | Metalloid | Radioactive Metal |
| Use in Lighting | Used in flame retardants and alloys for lamps | Used historically in gas mantles for bright white light |
| Light Emission | Non-luminous, indirect role | Strong luminescence in gas mantles |
| Toxicity | Moderate toxicity | Radioactive, high toxicity |
| Availability | Moderate, commercially available | Scarce, regulated due to radioactivity |
Introduction to Antimony and Thorium in Lighting
Antimony and thorium are both used in specialized lighting applications, with antimony primarily found in halide lamps for its ability to enhance light quality and color rendering. Thorium, historically used in gas mantles, provides high luminosity due to its radioactive properties, making it suitable for intense, long-lasting light sources. Advances in lighting technology have led to the replacement of thorium with safer alternatives, but its historical significance remains notable in the development of bright light illumination.
Historical Use of Antimony and Thorium in Lamps
Antimony and thorium have both played roles in the development of lighting technology, with thorium historically used in gas mantles due to its high incandescence and ability to produce bright white light. Antimony compounds, while less luminous, were utilized as stabilizers and opacifiers in lamp glasses and flame retardants, influencing durability rather than light emission. Thorium's radioactive properties were advantageous for efficient gas mantles, but safety concerns led to a decline in its use, whereas antimony remains valued for its chemical stability in lighting components.
Chemical Properties Relevant to Lighting Applications
Antimony exhibits strong electron affinity and stable oxidation states, making it effective as a dopant in semiconductor lighting devices, particularly in infrared LEDs. Thorium's high atomic number and radioactivity influence its chemical stability but limit its practical use in lighting due to safety concerns and regulatory restrictions. The contrasting chemical properties--Antimony's versatile valence states versus Thorium's radioactive nature--drive their respective roles in phosphor synthesis and energy emission efficiency in lighting technologies.
Efficiency: Antimony vs Thorium in Light Production
Antimony and thorium differ significantly in light production efficiency, with thorium offering superior luminous efficacy due to its ability to emit intense, stable light when used in gas mantles. Thorium-based mantles produce a higher color temperature and brighter illumination compared to antimony, which generally yields lower luminous intensity and efficiency. The radioactive properties of thorium contribute to a more consistent and long-lasting light output, making it preferable for applications requiring efficient and durable lighting solutions.
Safety and Health Considerations
Antimony-based lighting components pose toxicity risks due to antimony's classification as a carcinogen and its potential to cause respiratory and skin irritation upon prolonged exposure. Thorium, a weakly radioactive element, presents radiological hazards, requiring strict handling protocols to prevent inhalation or ingestion of dust and limiting long-term exposure to gamma radiation. While both substances necessitate safety measures, antimony's chemical toxicity contrasts with thorium's radiotoxicity, influencing regulatory standards and workplace health monitoring in lighting manufacturing.
Environmental Impact and Sustainability
Antimony is often used in flame retardants and as a component in some LED lighting, but its mining and refining processes release toxic pollutants, raising concerns about environmental contamination and human health risks. Thorium, a naturally occurring radioactive element, presents sustainability advantages in lighting applications by offering potential for long-lasting, low-waste energy production through thorium-based nuclear technologies. The environmental impact of thorium lighting is minimized by its lower greenhouse gas emissions compared to fossil fuels, while antimony's extraction contributes significantly to soil and water pollution, making thorium a more sustainable choice for future lighting innovations.
Cost and Availability for Industrial Uses
Antimony offers cost advantages due to its abundant supply and lower extraction expenses compared to Thorium, which is rarer and often subject to strict regulatory controls because of its radioactivity. Thorium's limited availability and higher processing costs make it less economically feasible for large-scale industrial lighting applications. Industries typically prefer Antimony in lighting components such as semiconductors and flame retardants, balancing performance with affordability and reliable material sourcing.
Performance Comparison in Modern Lighting Technologies
Antimony and Thorium differ significantly in their performance in modern lighting technologies, with Thorium-based phosphors offering higher luminous efficacy and better color rendering compared to Antimony compounds. Thorium's ability to emit stable, intense light under electrical excitation makes it advantageous for applications requiring bright, long-lasting illumination, while Antimony is often valued for its lower toxicity and cost-effectiveness. In terms of luminous efficiency and spectral quality, Thorium consistently outperforms Antimony, making it the preferred choice in high-performance lighting systems such as advanced fluorescent lamps and LEDs.
Regulatory Standards and Restrictions
Antimony usage in lighting is regulated under strict EU REACH standards due to its toxicity and environmental impact, limiting its permissible concentration in consumer products. Thorium, a radioactive element, faces extensive restrictions globally, including classifications under IAEA guidelines and bans in many countries for consumer lighting applications due to radiological health risks. Compliance with local hazardous materials regulations often dictates the choice between antimony-based phosphors and thorium-containing gas mantles in commercial and industrial lighting.
Future Prospects in Lighting Innovation
Thorium offers promising potential in lighting innovation due to its radioactive properties, enabling more efficient and longer-lasting light sources compared to antimony-based materials. Antimony remains valuable for its role in semiconductors and flame-retardant coatings but faces limitations in energy efficiency and lifespan when compared to emerging thorium applications. Future advancements in thorium-based lighting technologies could revolutionize energy consumption and durability standards in the illumination industry.
Infographic: Antimony vs Thorium for Lighting
 azmater.com
azmater.com