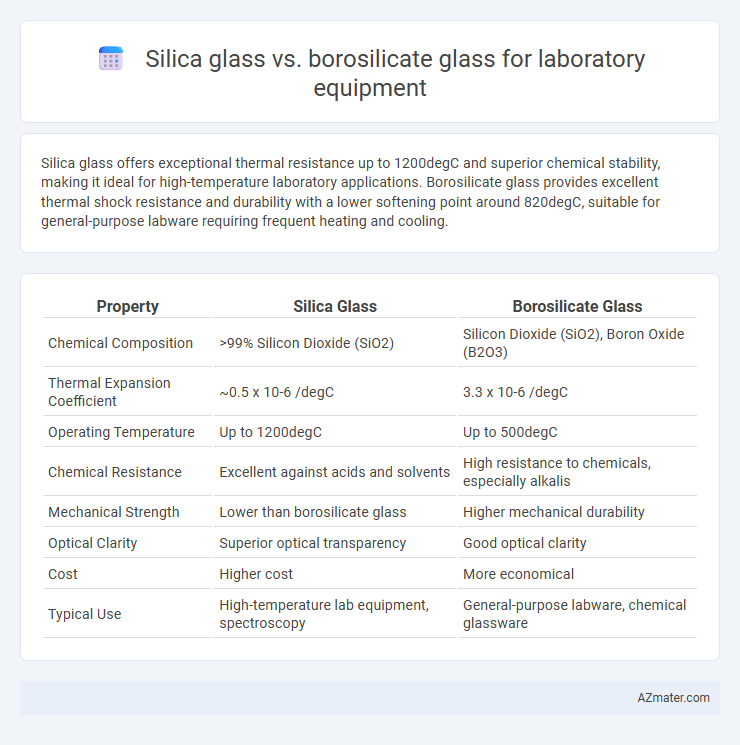
Silica glass offers exceptional thermal resistance up to 1200degC and superior chemical stability, making it ideal for high-temperature laboratory applications. Borosilicate glass provides excellent thermal shock resistance and durability with a lower softening point around 820degC, suitable for general-purpose labware requiring frequent heating and cooling.
Table of Comparison
| Property | Silica Glass | Borosilicate Glass |
|---|---|---|
| Chemical Composition | >99% Silicon Dioxide (SiO2) | Silicon Dioxide (SiO2), Boron Oxide (B2O3) |
| Thermal Expansion Coefficient | ~0.5 x 10-6 /degC | 3.3 x 10-6 /degC |
| Operating Temperature | Up to 1200degC | Up to 500degC |
| Chemical Resistance | Excellent against acids and solvents | High resistance to chemicals, especially alkalis |
| Mechanical Strength | Lower than borosilicate glass | Higher mechanical durability |
| Optical Clarity | Superior optical transparency | Good optical clarity |
| Cost | Higher cost | More economical |
| Typical Use | High-temperature lab equipment, spectroscopy | General-purpose labware, chemical glassware |
Introduction to Laboratory Glassware Materials
Silica glass and borosilicate glass are fundamental materials used in laboratory glassware, each offering distinct thermal and chemical properties. Silica glass, composed primarily of pure silicon dioxide, provides superior thermal resistance and optical clarity but is more brittle and expensive. Borosilicate glass contains boron trioxide, enhancing thermal shock resistance and chemical durability, making it the preferred choice for general-purpose laboratory applications such as beakers, flasks, and test tubes.
Chemical Composition of Silica and Borosilicate Glass
Silica glass primarily consists of silicon dioxide (SiO2) with a purity often exceeding 99.9%, providing excellent thermal stability and chemical resistance. Borosilicate glass contains about 70-80% silicon dioxide (SiO2), 7-13% boron oxide (B2O3), along with smaller amounts of aluminum oxide (Al2O3) and alkali metal oxides, which enhances its thermal shock resistance and chemical durability. The incorporation of boron oxide in borosilicate glass lowers its thermal expansion coefficient compared to pure silica glass, making it more resistant to sudden temperature changes in laboratory applications.
Thermal Resistance: Silica vs. Borosilicate Glass
Silica glass exhibits superior thermal resistance with a high softening point around 1713degC, making it ideal for extreme high-temperature laboratory applications. Borosilicate glass resists thermal shock well with a lower softening point near 820degC and a coefficient of thermal expansion around 3.3 x 10-6 /degC. Laboratories requiring durable glassware for rapid temperature changes often prefer borosilicate for its balance of thermal stability and resistance to thermal shock.
Chemical Durability and Corrosion Resistance
Silica glass exhibits exceptional chemical durability and corrosion resistance, making it highly resistant to most acids, alkalis, and organic solvents commonly used in laboratory environments. Borosilicate glass offers excellent resistance to thermal shock and moderate chemical corrosion, particularly against aqueous alkaline solutions and acids, due to its boron oxide content. For applications requiring sustained exposure to highly corrosive chemicals or extreme chemical purity, silica glass generally outperforms borosilicate glass in maintaining structural integrity and clarity over time.
Mechanical Strength and Durability
Silica glass offers exceptional mechanical strength and resistance to high temperatures, making it ideal for heavy-duty laboratory applications requiring thermal shock resistance and chemical inertness. Borosilicate glass, while slightly less strong, provides superior durability against thermal expansion and impact, ensuring reliable performance during rapid temperature changes. Both materials maintain excellent chemical resistance, but silica glass is preferred for ultra-high temperature stability, whereas borosilicate glass balances strength and durability for versatile laboratory use.
Optical Clarity and Light Transmission
Silica glass offers superior optical clarity and higher light transmission compared to borosilicate glass, making it ideal for precise optical measurements and high-sensitivity laboratory applications. Its low impurity levels result in minimal light absorption and excellent UV transmission, enhancing accuracy in spectroscopic and photometric analyses. Borosilicate glass provides good optical clarity but exhibits slightly lower transparency and more light scattering, which can limit its effectiveness in advanced optical experiments.
Cost Comparison for Laboratory Applications
Silica glass generally carries a higher cost than borosilicate glass due to its superior thermal resistance and chemical purity, making it ideal for high-temperature and highly corrosive laboratory applications. Borosilicate glass offers a more cost-effective option with excellent thermal shock resistance and durability suitable for routine lab use, balancing performance and budget constraints. Laboratories aiming for long-term investment in specialized applications may justify the premium price of silica glass, while borosilicate remains the preferred choice for everyday glassware due to its affordability.
Common Laboratory Uses for Silica Glass
Silica glass is preferred in laboratory equipment for high-temperature applications, such as furnaces and reactors, due to its exceptional thermal resistance up to 1,100degC. Its excellent chemical inertness makes it ideal for handling corrosive substances and precise analytical procedures, including spectroscopy and semiconductor manufacturing. Common laboratory uses include crucibles, heating tubes, and optical components where minimal thermal expansion and high purity are critical.
Typical Uses of Borosilicate Glass in Labs
Borosilicate glass is widely used in laboratory equipment due to its excellent thermal resistance and chemical durability, making it ideal for beakers, flasks, and test tubes that require repetitive heating and cooling cycles. Its low thermal expansion minimizes the risk of cracking under rapid temperature changes, making it suitable for applications involving high-temperature reactions and autoclaving. Borosilicate glass also resists chemical corrosion from acids and alkalis, which is essential for handling aggressive reagents in analytical and synthetic chemistry labs.
Choosing the Right Glass for Laboratory Equipment
Silica glass offers superior thermal resistance and chemical inertness, making it ideal for high-temperature experiments and applications involving aggressive chemicals. Borosilicate glass, characterized by its excellent thermal shock resistance and mechanical durability, is preferred for general laboratory use where frequent temperature changes occur. Selecting between silica and borosilicate glass depends on the specific thermal and chemical requirements of the laboratory procedures.
Infographic: Silica glass vs Borosilicate glass for Laboratory equipment
 azmater.com
azmater.com