Dichroic glass, known for its multi-layer thin-film coatings, offers superior optical properties and enhanced light reflection compared to Potassium glass, which primarily provides chemical resistance and thermal stability. Laboratory glassware made from Dichroic glass enables precise spectroscopic analysis, while Potassium glass excels in durability for high-temperature experiments.
Table of Comparison
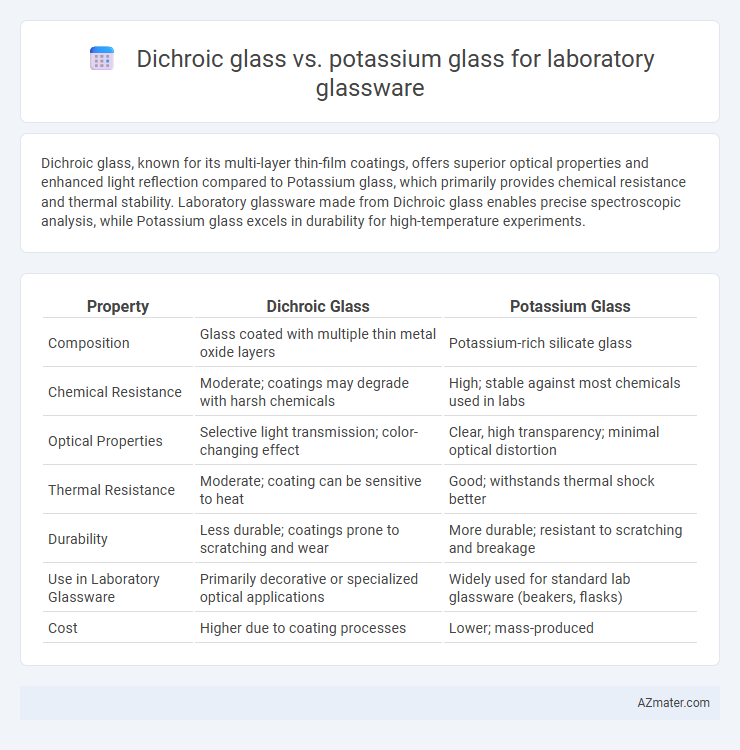
| Property | Dichroic Glass | Potassium Glass |
|---|---|---|
| Composition | Glass coated with multiple thin metal oxide layers | Potassium-rich silicate glass |
| Chemical Resistance | Moderate; coatings may degrade with harsh chemicals | High; stable against most chemicals used in labs |
| Optical Properties | Selective light transmission; color-changing effect | Clear, high transparency; minimal optical distortion |
| Thermal Resistance | Moderate; coating can be sensitive to heat | Good; withstands thermal shock better |
| Durability | Less durable; coatings prone to scratching and wear | More durable; resistant to scratching and breakage |
| Use in Laboratory Glassware | Primarily decorative or specialized optical applications | Widely used for standard lab glassware (beakers, flasks) |
| Cost | Higher due to coating processes | Lower; mass-produced |
Introduction to Laboratory Glassware Materials
Laboratory glassware materials primarily include dichroic glass and potassium glass, each selected for specific optical and chemical properties. Dichroic glass features thin-film coatings that enable selective light transmission, making it ideal for analytical instruments requiring precise wavelength filtering. Potassium glass, with its high resistance to chemical corrosion and thermal stability, is favored for general-purpose glassware such as beakers and flasks in chemical laboratories.
Overview of Dichroic Glass
Dichroic glass is a specialized material characterized by its ability to selectively reflect and transmit light, creating vibrant color effects due to thin-film interference coatings. This unique optical property makes dichroic glass highly valuable in laboratory glassware where precise light manipulation is required, such as in optical filters and sensors. Compared to potassium glass, dichroic glass offers enhanced spectral control and durability, but it typically comes at a higher cost and complexity of manufacture.
Overview of Potassium Glass
Potassium glass, known for its high chemical durability and resistance to thermal shock, is widely used in laboratory glassware requiring robust performance under extreme conditions. This type of glass contains potassium oxide, which enhances its mechanical strength and lowers thermal expansion compared to standard soda-lime glass, making it ideal for precision laboratory instruments. Its superior chemical resistance ensures minimal interaction with reactive substances, maintaining purity and reliability in experimental results.
Chemical Resistance Comparison
Dichroic glass exhibits moderate chemical resistance, making it suitable for general laboratory applications but less effective against strong acids and alkalis compared to Potassium glass. Potassium glass offers superior resistance to chemical corrosion, particularly from aggressive substances like hydrofluoric acid and strong alkaline solutions, enhancing its durability in harsh chemical environments. Choosing between Dichroic and Potassium glass hinges on the specific chemical exposure and required longevity of the laboratory glassware.
Thermal Stability and Heat Resistance
Dichroic glass, known for its multi-layer optical coatings, generally offers moderate thermal stability but lower heat resistance compared to potassium glass, which contains potassium oxide to enhance its thermal shock resistance and high-temperature durability. Potassium glass is commonly preferred in laboratory glassware requiring frequent exposure to rapid temperature changes due to its superior ability to withstand thermal stress without cracking. In applications demanding both optical properties and heat tolerance, the choice depends on prioritizing either thermal performance (potassium glass) or specific light-filtering characteristics (dichroic glass).
Optical Properties and Light Transmission
Dichroic glass features multiple ultra-thin layers of metal oxides that selectively filter and reflect specific wavelengths, resulting in unique color-shifting optical effects and high spectral selectivity, making it ideal for advanced optical applications. Potassium glass, composed primarily of potassium oxide and silica, offers high refractive index and excellent light transmission with minimal color distortion, suitable for precise laboratory measurements requiring clarity and durability. The choice between dichroic and potassium glass depends on specific optical needs: dichroic glass excels in wavelength-specific filtration, while potassium glass provides consistent broadband light transmission with enhanced chemical resistance.
Durability and Mechanical Strength
Dichroic glass used in laboratory glassware exhibits enhanced optical properties but generally offers lower durability and mechanical strength compared to potassium glass. Potassium glass, a type of borosilicate glass, demonstrates superior resistance to thermal shock and chemical corrosion, making it more suitable for rigorous laboratory applications. The higher mechanical strength and fracture toughness of potassium glass ensure longer lifespan and reliability under repeated thermal and mechanical stress.
Applications in Laboratory Settings
Dichroic glass is primarily used in laboratory settings for optical filters and specialized microscopy due to its unique wavelength-selective properties, enhancing fluorescence imaging and light separation. Potassium glass, known for its chemical resistance and durability, is favored in standard laboratory glassware such as beakers and test tubes for handling corrosive substances and high-temperature reactions. Each glass type's unique physical and chemical properties dictate its optimal application in laboratories, with dichroic glass excelling in precision optical instrumentation and potassium glass delivering robust performance in routine chemical processing.
Cost and Availability Considerations
Dichroic glass laboratoryware tends to be significantly more expensive due to its specialized coating process and limited availability compared to potassium glass, which is widely produced and more affordable. Potassium glass offers greater accessibility and cost-effectiveness for routine laboratory applications, while dichroic glass is typically reserved for niche uses requiring specific optical properties. Availability of dichroic glass is often restricted to specialized suppliers, whereas potassium glass is readily stocked by most standard laboratory glassware vendors.
Choosing the Right Glass for Laboratory Use
Dichroic glass offers superior optical properties and color-changing effects due to thin-film coatings, making it ideal for specialized laboratory applications requiring precise light manipulation or visual indicators. Potassium glass, with its high chemical resistance and durability, is better suited for general-purpose laboratory glassware exposed to strong chemicals and thermal stress. Selecting the right glass depends on the specific laboratory needs--optical performance favors dichroic glass, while chemical stability and robustness prioritize potassium glass.
Infographic: Dichroic glass vs Potassium glass for Laboratory glassware
 azmater.com
azmater.com