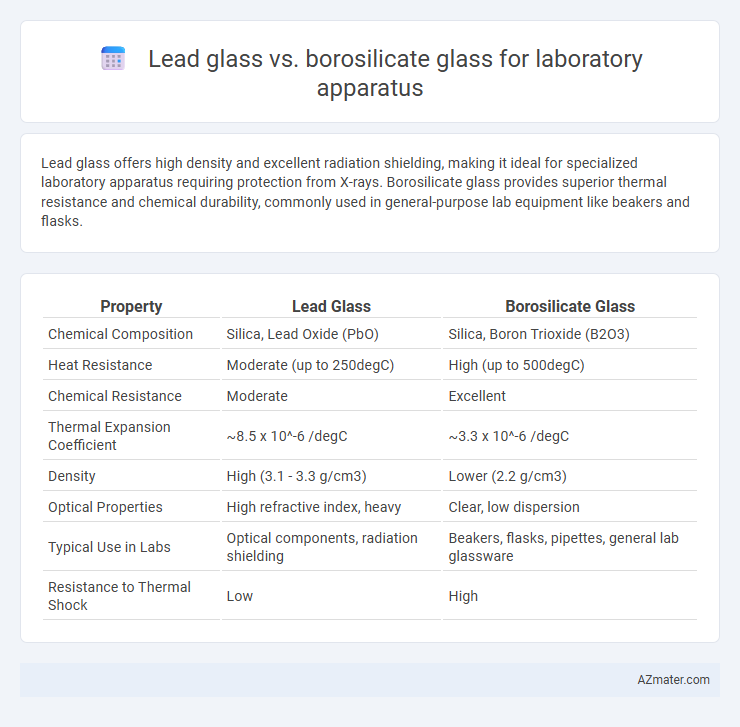
Lead glass offers high density and excellent radiation shielding, making it ideal for specialized laboratory apparatus requiring protection from X-rays. Borosilicate glass provides superior thermal resistance and chemical durability, commonly used in general-purpose lab equipment like beakers and flasks.
Table of Comparison
| Property | Lead Glass | Borosilicate Glass |
|---|---|---|
| Chemical Composition | Silica, Lead Oxide (PbO) | Silica, Boron Trioxide (B2O3) |
| Heat Resistance | Moderate (up to 250degC) | High (up to 500degC) |
| Chemical Resistance | Moderate | Excellent |
| Thermal Expansion Coefficient | ~8.5 x 10^-6 /degC | ~3.3 x 10^-6 /degC |
| Density | High (3.1 - 3.3 g/cm3) | Lower (2.2 g/cm3) |
| Optical Properties | High refractive index, heavy | Clear, low dispersion |
| Typical Use in Labs | Optical components, radiation shielding | Beakers, flasks, pipettes, general lab glassware |
| Resistance to Thermal Shock | Low | High |
Introduction to Laboratory Glass Types
Lead glass and borosilicate glass are essential materials used in laboratory apparatus due to their unique chemical and thermal properties. Borosilicate glass offers high thermal resistance and chemical durability, making it ideal for heating and chemical experiments, while lead glass, containing lead oxide, provides superior clarity and density but lower thermal resistance. Selecting the appropriate glass type depends on the specific laboratory application requirements, including thermal stability, chemical compatibility, and optical properties.
Composition of Lead Glass
Lead glass contains a high proportion of lead oxide, typically between 18% and 40%, which enhances its density and refractive index, making it ideal for optical clarity in laboratory apparatus. In contrast, borosilicate glass is composed mainly of silica and boron trioxide, offering superior thermal resistance and chemical durability but lower density. The lead content in lead glass also contributes to improved radiation shielding properties, beneficial for certain lab applications requiring protection from X-rays or gamma rays.
Composition of Borosilicate Glass
Borosilicate glass, primarily composed of silica (SiO2) and boron trioxide (B2O3), offers superior thermal resistance and chemical durability compared to lead glass, which contains significant amounts of lead oxide (PbO) for increased refractive index but lower thermal stability. The incorporation of boron trioxide in borosilicate glass reduces thermal expansion, making it ideal for laboratory apparatus that undergo rapid temperature changes. This composition enables borosilicate glass to resist chemical corrosion and mechanical stress, ensuring longer lifespan and safety in scientific applications.
Thermal Properties Comparison
Lead glass exhibits lower thermal expansion coefficients, typically around 3.3 x 10-6 /degC, enhancing its resistance to thermal shock compared to borosilicate glass, which has a coefficient near 3.3 x 10-6 /degC but generally offers higher chemical durability. Borosilicate glass withstands temperatures up to approximately 560degC with excellent thermal stability, while lead glass tolerates lower maximum temperatures, often below 400degC. These thermal properties make borosilicate glass more suitable for rapid temperature changes and high-temperature laboratory applications.
Chemical Resistance: Lead vs Borosilicate Glass
Borosilicate glass exhibits superior chemical resistance compared to lead glass, making it the preferred choice for laboratory apparatus exposed to strong acids and alkalis. Lead glass tends to be more susceptible to chemical attack, especially by alkaline solutions, which can degrade its surface and compromise durability. The enhanced chemical stability of borosilicate glass ensures longer lifespan and reliability under harsh laboratory conditions.
Optical Clarity and Transparency Differences
Lead glass exhibits superior optical clarity and higher refractive index compared to borosilicate glass, resulting in enhanced light transmission and brilliance ideal for precision optical applications. Borosilicate glass offers excellent transparency with minimal color distortion and exceptional thermal resistance, maintaining optical properties under rapid temperature changes common in laboratory environments. The choice between lead glass and borosilicate glass depends on the required balance between optical performance and durability against thermal and chemical stresses.
Safety and Toxicity Concerns
Lead glass contains significant amounts of lead oxide, which poses toxicity risks if the glass is damaged or improperly handled, releasing lead particles or ions that can contaminate laboratory environments and samples. Borosilicate glass is favored for laboratory apparatus due to its chemical inertness and minimal toxicity, reducing health hazards associated with exposure to harmful substances. Safety concerns with lead glass include potential lead poisoning and environmental contamination, while borosilicate glass offers superior safety profiles in terms of both human health and environmental impact.
Durability and Mechanical Strength
Borosilicate glass exhibits superior durability and mechanical strength compared to lead glass, making it highly resistant to thermal shock and mechanical stress in laboratory environments. Lead glass, while offering excellent optical clarity, is softer and more prone to scratching and breakage under rigorous use. Laboratories prioritize borosilicate glass for apparatus due to its enhanced toughness and ability to withstand repeated heating and cooling cycles without cracking.
Cost and Availability Considerations
Lead glass typically costs more than borosilicate glass due to its higher lead content and specialized manufacturing processes, impacting laboratory budgets. Borosilicate glass is more widely available and economically favorable because of its extensive industrial use and simpler production, making it the preferred choice for standard laboratory apparatus. Availability and cost-efficiency make borosilicate glass the dominant option in laboratory settings where large-scale procurement is necessary.
Applications and Best Use Cases in Laboratories
Lead glass is preferred in laboratory applications requiring high radiation shielding and enhanced optical clarity, such as in radiation detection and precision spectroscopy. Borosilicate glass excels in thermal resistance and chemical durability, making it ideal for high-temperature reactions, distillation, and storage of aggressive chemicals. Laboratories benefit from choosing lead glass for protective viewing windows and borosilicate glass for autoclaving and corrosive environment experiments.
Infographic: Lead glass vs Borosilicate glass for Laboratory apparatus
 azmater.com
azmater.com