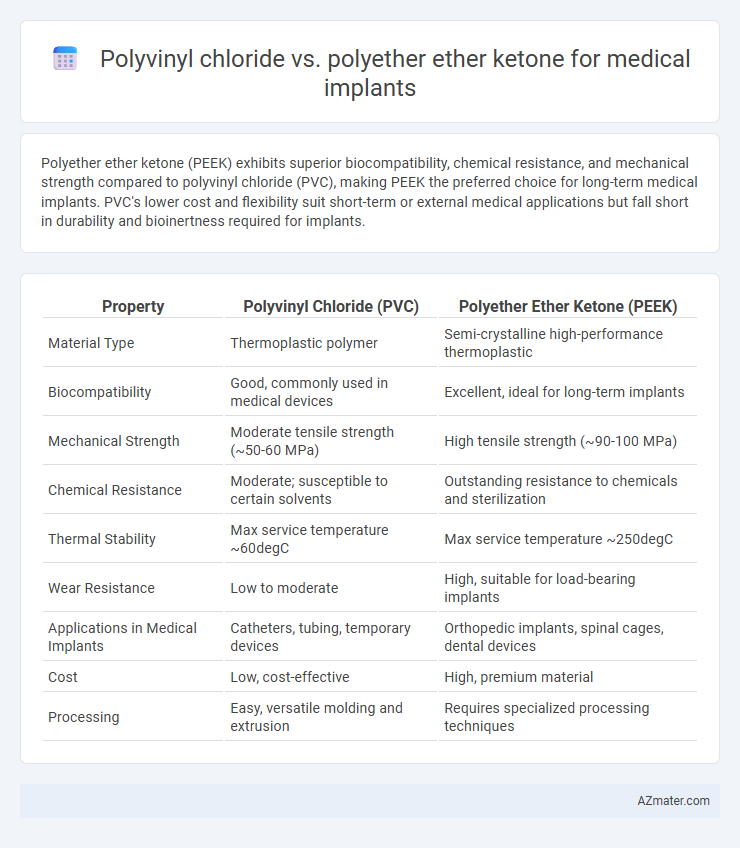
Polyether ether ketone (PEEK) exhibits superior biocompatibility, chemical resistance, and mechanical strength compared to polyvinyl chloride (PVC), making PEEK the preferred choice for long-term medical implants. PVC's lower cost and flexibility suit short-term or external medical applications but fall short in durability and bioinertness required for implants.
Table of Comparison
| Property | Polyvinyl Chloride (PVC) | Polyether Ether Ketone (PEEK) |
|---|---|---|
| Material Type | Thermoplastic polymer | Semi-crystalline high-performance thermoplastic |
| Biocompatibility | Good, commonly used in medical devices | Excellent, ideal for long-term implants |
| Mechanical Strength | Moderate tensile strength (~50-60 MPa) | High tensile strength (~90-100 MPa) |
| Chemical Resistance | Moderate; susceptible to certain solvents | Outstanding resistance to chemicals and sterilization |
| Thermal Stability | Max service temperature ~60degC | Max service temperature ~250degC |
| Wear Resistance | Low to moderate | High, suitable for load-bearing implants |
| Applications in Medical Implants | Catheters, tubing, temporary devices | Orthopedic implants, spinal cages, dental devices |
| Cost | Low, cost-effective | High, premium material |
| Processing | Easy, versatile molding and extrusion | Requires specialized processing techniques |
Introduction to Polymer Materials in Medical Implants
Polyvinyl chloride (PVC) and polyether ether ketone (PEEK) represent two contrasting polymer materials widely used in medical implants due to their unique properties. PVC offers versatile chemical resistance and cost-effectiveness, making it suitable for temporary implants and tubing, whereas PEEK provides superior mechanical strength, biocompatibility, and resistance to sterilization processes, ideal for long-term orthopedic and spinal implants. The selection of polymer materials in medical implants depends on factors such as biostability, mechanical performance, and compatibility with human tissue, with PEEK increasingly favored for high-performance and durable implant applications.
Overview of Polyvinyl Chloride (PVC)
Polyvinyl Chloride (PVC) is a widely used thermoplastic polymer noted for its versatility, biocompatibility, and cost-effectiveness, making it a common choice for medical implants such as tubing and blood bags. Its inherent resistance to chemicals, flexibility, and ease of sterilization contribute to its suitability in various medical applications, though concerns regarding plasticizer migration and long-term stability exist. PVC's mechanical properties and ability to be tailored through additives position it as a practical material for temporary or short-term medical devices compared to high-performance polymers like Polyether ether ketone (PEEK).
Overview of Polyether Ether Ketone (PEEK)
Polyether ether ketone (PEEK) is a high-performance thermoplastic with exceptional biocompatibility, chemical resistance, and mechanical strength, making it ideal for medical implants. Unlike polyvinyl chloride (PVC), PEEK offers superior fatigue resistance and thermal stability, enabling long-term durability within the human body. Its radiolucency and minimal inflammatory response enhance its suitability for orthopedic and dental implant applications.
Biocompatibility: PVC vs. PEEK
Polyether ether ketone (PEEK) outperforms polyvinyl chloride (PVC) in biocompatibility for medical implants due to its inert chemical structure, which minimizes adverse tissue reactions and inflammation. PVC often releases plasticizers and additives that can cause cytotoxicity and long-term biocompatibility concerns in implantable devices. PEEK's proven success in orthopedic and spinal implants highlights its superior compatibility with human tissue compared to the more reactive nature of PVC.
Mechanical Properties Comparison
Polyether ether ketone (PEEK) exhibits superior mechanical properties compared to polyvinyl chloride (PVC) for medical implants, including higher tensile strength (90-100 MPa for PEEK vs. 30-50 MPa for PVC) and greater impact resistance. PEEK's excellent fatigue resistance and modulus of elasticity (3.6 GPa) provide enhanced load-bearing capabilities, making it suitable for orthopedic and spinal implants. In contrast, PVC has lower biocompatibility and mechanical stability, limiting its use in long-term implant applications.
Sterilization and Chemical Resistance
Polyether ether ketone (PEEK) exhibits superior chemical resistance and maintains mechanical integrity after repeated sterilization cycles, making it highly suitable for medical implants requiring frequent autoclaving or exposure to harsh disinfectants. Polyvinyl chloride (PVC), while cost-effective and flexible, shows limited resistance to high-temperature sterilization methods and can degrade when exposed to certain sterilizing chemicals, posing potential biocompatibility risks. The choice between PEEK and PVC for medical implants hinges on PEEK's enhanced durability during sterilization and chemical exposure, crucial for long-term implant performance.
Long-term Performance in the Body
Polyether ether ketone (PEEK) demonstrates superior long-term biostability and mechanical integrity compared to polyvinyl chloride (PVC) for medical implants, resisting hydrolytic and enzymatic degradation within the body. PEEK's excellent chemical resistance and minimal inflammatory response promote sustained implant functionality, while PVC often suffers from plasticizer leaching and reduced structural performance over time. The enhanced durability and biocompatibility of PEEK make it a preferred choice for load-bearing and long-term implant applications in orthopedic and cardiovascular devices.
Safety and Regulatory Approvals
Polyvinyl chloride (PVC) is widely used in medical implants due to its cost-effectiveness and flexibility but poses safety concerns related to plasticizer leaching and potential toxic effects, restricting its use in long-term implants under stringent regulatory approvals such as FDA and CE marking. Polyether ether ketone (PEEK) offers superior biocompatibility, chemical resistance, and mechanical stability, making it favorable for permanent implants with consistent regulatory clearance for safety in orthopedic and spinal applications. Regulatory bodies prefer PEEK over PVC for implantable devices due to its inert nature and proven track record in minimizing adverse inflammatory responses and ensuring patient safety.
Typical Applications in Medical Implants
Polyvinyl chloride (PVC) is commonly used in medical implants such as tubing, catheter components, and blood bags due to its flexibility, biocompatibility, and cost-effectiveness. Polyether ether ketone (PEEK) is preferred for load-bearing implants like spinal cages, orthopedic devices, and dental implants because of its superior mechanical strength, chemical resistance, and bioinertness. PVC excels in temporary, flexible applications, while PEEK is chosen for permanent, high-performance implants requiring durability and stability.
Cost Analysis and Market Trends
Polyvinyl chloride (PVC) remains a cost-effective choice for medical implants due to its low material price and ease of manufacturing, driving high market adoption in budget-sensitive applications. Polyether ether ketone (PEEK) offers superior biocompatibility, mechanical strength, and chemical resistance but entails higher production costs, limiting its use to premium, high-performance implant markets. Market trends indicate increasing demand for PEEK in advanced orthopedic and dental implants, driven by growing preference for long-term durability and regulatory push for biocompatible materials despite its higher cost premium compared to PVC.
Infographic: Polyvinyl chloride vs Polyether ether ketone for Medical Implant
 azmater.com
azmater.com