Polyether Ether Ketone (PEEK) offers superior biocompatibility, chemical resistance, and mechanical strength compared to Polyoxymethylene (POM), making it a preferred choice for durable and long-term medical implants. POM, while cost-effective and rigid, lacks the high-temperature stability and sterilization versatility required for advanced implant applications.
Table of Comparison
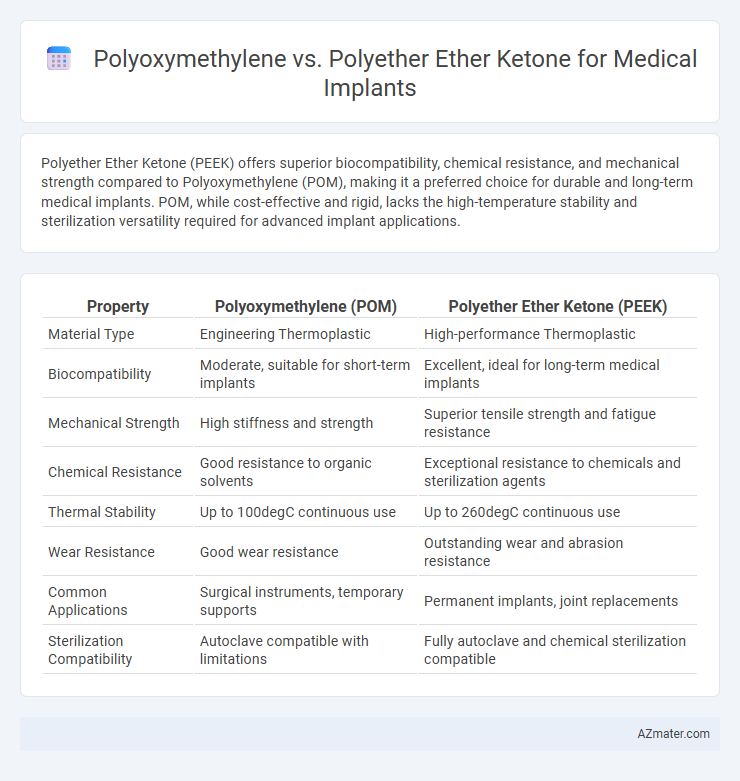
| Property | Polyoxymethylene (POM) | Polyether Ether Ketone (PEEK) |
|---|---|---|
| Material Type | Engineering Thermoplastic | High-performance Thermoplastic |
| Biocompatibility | Moderate, suitable for short-term implants | Excellent, ideal for long-term medical implants |
| Mechanical Strength | High stiffness and strength | Superior tensile strength and fatigue resistance |
| Chemical Resistance | Good resistance to organic solvents | Exceptional resistance to chemicals and sterilization agents |
| Thermal Stability | Up to 100degC continuous use | Up to 260degC continuous use |
| Wear Resistance | Good wear resistance | Outstanding wear and abrasion resistance |
| Common Applications | Surgical instruments, temporary supports | Permanent implants, joint replacements |
| Sterilization Compatibility | Autoclave compatible with limitations | Fully autoclave and chemical sterilization compatible |
Introduction to Polyoxymethylene and Polyether Ether Ketone
Polyoxymethylene (POM), also known as acetal, is a high-strength, low-friction engineering thermoplastic commonly used in medical implants for its excellent dimensional stability and biocompatibility. Polyether Ether Ketone (PEEK) is a semicrystalline thermoplastic renowned for its outstanding chemical resistance, mechanical strength, and radiolucency, making it ideal for long-term medical implant applications. Both polymers provide unique advantages in orthopedic and dental implants, where durability, sterilizability, and biological inertness are critical.
Chemical Structure and Properties Comparison
Polyoxymethylene (POM) features a repeating -CH2-O- structure that provides high crystallinity, excellent stiffness, and wear resistance, making it suitable for rigid medical implants. Polyether Ether Ketone (PEEK) contains aromatic rings linked by ether and ketone groups, giving it superior chemical resistance, thermal stability up to 250degC, and biocompatibility for long-term implant applications. The semi-crystalline nature of POM results in lower thermal resistance compared to the aromatic, high-performance polymer PEEK, which also exhibits enhanced fatigue resistance essential for durable implant performance.
Mechanical Strength and Durability
Polyether Ether Ketone (PEEK) offers superior mechanical strength and exceptional durability compared to Polyoxymethylene (POM) for medical implant applications. PEEK exhibits high tensile strength, excellent fatigue resistance, and stability under sterilization processes, making it ideal for long-term implant performance. In contrast, POM's lower mechanical robustness and susceptibility to hydrolysis limit its use in load-bearing medical implants.
Biocompatibility and Safety Profiles
Polyether ether ketone (PEEK) demonstrates superior biocompatibility and chemical resistance compared to polyoxymethylene (POM), making it a preferred choice for long-term medical implants due to its stability and low inflammatory response. PEEK's inherent radiolucency and resistance to sterilization methods such as gamma irradiation enhance its safety profile, whereas POM may show degradation or bio-incompatibility risks under similar conditions. The high mechanical strength and excellent wear resistance of PEEK also reduce implant failure and ensure longevity in demanding biomedical applications.
Sterilization Capability and Resistance
Polyether Ether Ketone (PEEK) demonstrates superior sterilization capability and chemical resistance compared to Polyoxymethylene (POM), making it more suitable for medical implants exposed to aggressive sterilization methods like autoclaving and gamma radiation. PEEK maintains mechanical integrity and dimensional stability under repeated sterilization cycles, while POM tends to degrade or warp due to lower thermal resistance and susceptibility to hydrolysis. These properties position PEEK as the preferred polymer in demanding medical environments requiring high sterilization endurance and biocompatibility.
Wear and Corrosion Resistance
Polyether Ether Ketone (PEEK) exhibits superior wear and corrosion resistance compared to Polyoxymethylene (POM), making it a preferred choice for long-term medical implants exposed to bodily fluids and mechanical stress. PEEK's inherent chemical stability and high mechanical strength minimize degradation and wear debris formation, reducing inflammation risks. In contrast, POM shows higher susceptibility to hydrolytic degradation and wear under cyclic loading, limiting its durability in implant applications.
Applications in Medical Implants
Polyoxymethylene (POM) offers excellent mechanical strength and dimensional stability, making it suitable for medical implants such as orthopedic devices and surgical instruments that require rigidity and fatigue resistance. Polyether Ether Ketone (PEEK) stands out for its superior biocompatibility, chemical resistance, and ability to withstand high sterilization temperatures, making it ideal for spinal implants, dental implants, and cranial plates. PEEK's radiolucency also allows for better imaging during postoperative evaluations, whereas POM is generally limited to applications where mechanical performance is prioritized over imaging compatibility.
Cost-effectiveness and Availability
Polyoxymethylene (POM) offers cost-effective manufacturing with widespread availability, making it suitable for non-critical medical implant components requiring good mechanical properties and chemical resistance. Polyether Ether Ketone (PEEK) commands a higher price due to superior biocompatibility, thermal stability, and mechanical strength, justifying its use in demanding load-bearing implants despite limited supplier options. Balancing cost-effectiveness and availability, POM fits budget-sensitive applications, while PEEK excels in advanced implants where performance outweighs expense.
Regulatory Approvals and Compliance
Polyether Ether Ketone (PEEK) boasts extensive regulatory approvals for medical implants, including FDA clearance and ISO 13485 certification, due to its excellent biocompatibility and chemical resistance. Polyoxymethylene (POM) has limited approvals in the medical field, primarily for non-implant devices, as it presents higher risks of degradation and biocompatibility challenges. Compliance with stringent medical device standards favors PEEK for long-term implant applications over POM, ensuring patient safety and regulatory adherence.
Conclusion: Choosing Between POM and PEEK for Implants
Polyether Ether Ketone (PEEK) offers superior biocompatibility, chemical resistance, and mechanical strength compared to Polyoxymethylene (POM), making it the preferred choice for long-term medical implants requiring durability and stability. Polyoxymethylene, while cost-effective and easy to machine, lacks the necessary wear resistance and inertness for high-performance implant applications. Selecting PEEK ensures enhanced patient safety and implant longevity, critical factors in medical device innovation.
Infographic: Polyoxymethylene vs Polyether Ether Ketone for Medical Implant
 azmater.com
azmater.com