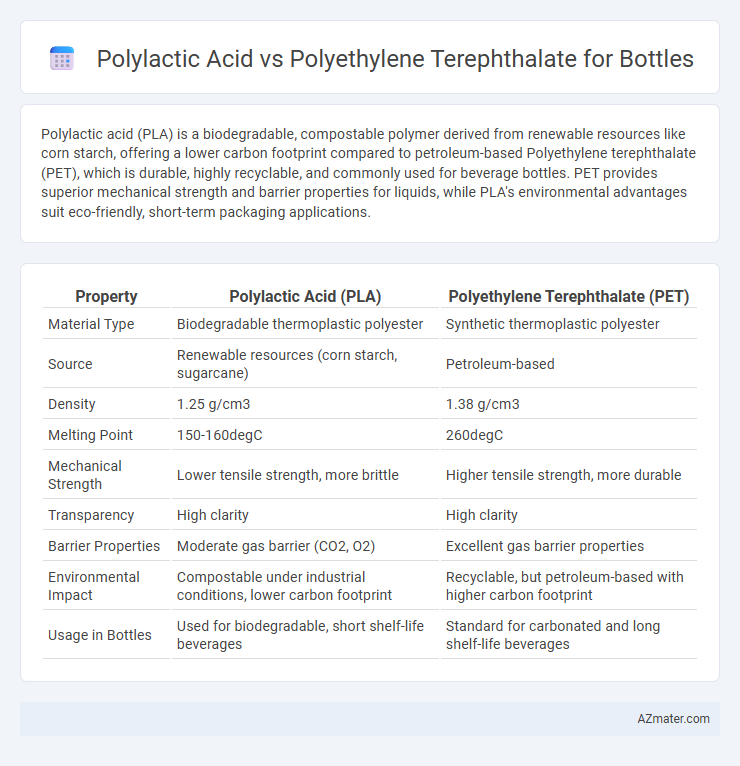
Polylactic acid (PLA) is a biodegradable, compostable polymer derived from renewable resources like corn starch, offering a lower carbon footprint compared to petroleum-based Polyethylene terephthalate (PET), which is durable, highly recyclable, and commonly used for beverage bottles. PET provides superior mechanical strength and barrier properties for liquids, while PLA's environmental advantages suit eco-friendly, short-term packaging applications.
Table of Comparison
| Property | Polylactic Acid (PLA) | Polyethylene Terephthalate (PET) |
|---|---|---|
| Material Type | Biodegradable thermoplastic polyester | Synthetic thermoplastic polyester |
| Source | Renewable resources (corn starch, sugarcane) | Petroleum-based |
| Density | 1.25 g/cm3 | 1.38 g/cm3 |
| Melting Point | 150-160degC | 260degC |
| Mechanical Strength | Lower tensile strength, more brittle | Higher tensile strength, more durable |
| Transparency | High clarity | High clarity |
| Barrier Properties | Moderate gas barrier (CO2, O2) | Excellent gas barrier properties |
| Environmental Impact | Compostable under industrial conditions, lower carbon footprint | Recyclable, but petroleum-based with higher carbon footprint |
| Usage in Bottles | Used for biodegradable, short shelf-life beverages | Standard for carbonated and long shelf-life beverages |
Introduction to Polylactic Acid (PLA) and Polyethylene Terephthalate (PET)
Polylactic Acid (PLA) is a biodegradable thermoplastic derived from renewable resources such as corn starch or sugarcane, making it an eco-friendly alternative in bottle manufacturing. Polyethylene Terephthalate (PET) is a petroleum-based polymer widely used for its strong, lightweight, and recyclable properties in beverage containers. Both PLA and PET offer distinct advantages in sustainability and performance, influencing packaging choices in the beverage industry.
Chemical Structure and Material Properties
Polylactic acid (PLA) is a biodegradable polyester derived from renewable resources like corn starch, consisting of repeating lactic acid units with ester linkages, offering a lower glass transition temperature around 60degC and moderate tensile strength. Polyethylene terephthalate (PET) is a petroleum-based polyester characterized by aromatic terephthalate and ethylene glycol units, providing higher thermal stability with a glass transition temperature near 70-80degC and superior mechanical strength. PLA exhibits brittleness and lower barrier properties compared to PET, which offers excellent gas barrier performance and durability, making PET more suitable for long-term liquid storage in bottles.
Environmental Impact: PLA vs PET
Polylactic acid (PLA) bottles offer a significant environmental advantage due to their biodegradability and production from renewable resources like corn starch, reducing reliance on fossil fuels. In contrast, polyethylene terephthalate (PET) is derived from non-renewable petroleum and, while highly recyclable, it persists in the environment for hundreds of years, contributing to plastic pollution. LCA studies indicate PLA bottles generally generate lower carbon emissions and have a smaller ecological footprint, though end-of-life composting infrastructure remains a critical factor in maximizing PLA's environmental benefits.
Production and Manufacturing Processes
Polylactic acid (PLA) production involves fermenting renewable resources like corn starch or sugarcane to produce lactic acid, followed by polymerization into PLA resin through condensation or ring-opening polymerization, making it a biodegradable alternative. Polyethylene terephthalate (PET) is synthesized via polycondensation of purified terephthalic acid (PTA) and ethylene glycol through a multi-step process involving esterification and polycondensation, relying heavily on petrochemical raw materials. PLA manufacturing requires lower energy consumption and emits less greenhouse gases compared to PET, which demands high-temperature melt processing and sophisticated crystallization for bottle-grade production.
Biodegradability and Compostability Comparison
Polylactic acid (PLA) is a biodegradable and compostable polymer derived from renewable resources like corn starch, breaking down into water and carbon dioxide under industrial composting conditions within 6 months. In contrast, Polyethylene terephthalate (PET) is a petroleum-based plastic that is not biodegradable or compostable, persisting in the environment for hundreds of years without natural breakdown. PLA bottles significantly reduce environmental impact through compostability, while PET bottles require recycling processes or contribute to long-term plastic pollution.
Mechanical Strength and Durability for Bottles
Polylactic acid (PLA) offers moderate mechanical strength with a tensile strength of around 50-70 MPa, but its brittleness limits impact resistance compared to Polyethylene terephthalate (PET), which boasts higher tensile strength of approximately 55-75 MPa and superior toughness. PET demonstrates excellent durability with strong resistance to environmental stress cracking and high flexibility, ensuring long-lasting performance for bottles subjected to pressure and handling. The biodegradability of PLA presents environmental advantages, yet PET's mechanical robustness and durability remain preferred for applications requiring extended shelf life and repeated use.
Food Safety and Migration Concerns
Polylactic acid (PLA) and Polyethylene terephthalate (PET) exhibit distinct differences in food safety and migration profiles for bottle applications. PLA, derived from renewable resources, generally has lower migration rates of harmful substances but may degrade faster under heat, potentially affecting food quality. PET is highly resistant to chemical migration and thermal degradation, making it a preferred choice for long-term food storage due to its proven safety and stability in contact with consumables.
Cost Analysis: PLA Bottles vs PET Bottles
Polylactic acid (PLA) bottles typically incur higher production costs compared to polyethylene terephthalate (PET) bottles due to the expensive fermentation process and raw materials like corn starch or sugarcane used in PLA. PET bottles benefit from economies of scale and lower material costs, making them more cost-effective for mass production. Despite higher initial expenses, PLA offers potential long-term savings with growing demand for biodegradable packaging and fluctuating fossil fuel prices affecting PET.
Recycling and Waste Management Challenges
Polylactic acid (PLA) and Polyethylene terephthalate (PET) differ significantly in recycling efficiency and waste management practices. PET benefits from established mechanical recycling systems, enabling high-quality material recovery and widespread reuse, whereas PLA's compostable nature complicates recycling streams due to contamination risks and the need for industrial composting facilities. Effective waste management of PLA requires specialized infrastructure to prevent landfill accumulation, while PET's recyclability supports circular economy goals by reducing environmental impact and resource consumption.
Future Trends and Market Adoption
Polylactic acid (PLA) is gaining traction in the bottle market due to its biodegradability and renewable sourcing, aligning with increasing environmental regulations and consumer demand for sustainable packaging. Polyethylene terephthalate (PET) maintains dominance because of its superior barrier properties, recyclability, and established recycling infrastructure, yet faces scrutiny over fossil fuel reliance and microplastic concerns. Future trends indicate a hybrid approach with enhanced PLA formulations and chemical recycling technologies for PET to balance performance, cost, and environmental impact, driving broader market adoption across beverage and personal care sectors.
Infographic: Polylactic acid vs Polyethylene terephthalate for Bottle
 azmater.com
azmater.com